7. COVID-19 Vaccines
Summary:
- The cumulative evidence pertaining to COVID-19 including the low infection fatality rate, the questionable efficacy of the vaccines, the VAERS and other data pertaining to reported adverse events, and the presence of proven alternative treatments, in addition to the violations of the established standard protocol for the testing of efficacy and safety of any treatment given to humans, all point to substantial violations of the various established protections of humans in research and outlined first in the Nuremberg Code, and reflect a significant attack on human dignity and human rights.
- The use of the vaccines is questionable for adults for the reasons outlined above. The current evidence indicates that the administration of the vaccines should be contraindicated in pregnant women and younger people.
- Efficacy of vaccines appears to be lower than natural immunity and increases the potential for severe infections from variants relative to those who are unvaccinated.
“Over One Thousand Scientific Studies Prove That the COVID-19 Vaccines Are Dangerous, and All Those Pushing This Agenda Are Committing the Indictable Crime of Gross Misconduct in Public Office”
“The statistically significant and overwhelmingly positive causal impact after vaccine deployment on the dependent variables total deaths and total cases per million should be highly worrisome for policy makers. They indicate a marked increase in both COVID-19 related cases and death due directly to a vaccine deployment that was originally sold to the public as the “key to gain back our freedoms.” (Beattie, 2021)
“In this review we first describe the technology underlying these vaccines [COVID-19 vaccines] in detail. We then review both components of and the intended biological response to these vaccines, including production of the spike protein itself, and their potential relationship to a wide range of both acute and long-term induced pathologies, such as blood disorders, neurodegenerative diseases and autoimmune diseases. Among these potential induced pathologies, we discuss the relevance of prion-protein-related amino acid sequences within the spike protein. We also present a brief review of studies supporting the potential for spike protein “shedding”, transmission of the protein from a vaccinated to an unvaccinated person, resulting in symptoms induced in the latter. We finish by addressing a common point of debate, namely, whether or not these vaccines could modify the DNA of those receiving the vaccination. While there are no studies demonstrating definitively that this is happening, we provide a plausible scenario, supported by previously established pathways for transformation and transport of genetic material, whereby injected mRNA could ultimately be incorporated into germ cell DNA for transgenerational transmission.” (Seneff and Nigh, 2021) [Comment: These authors address many concerns that are now coming to light and addressed in other parts of this document. The authors also conclude with a number of important, legitimate recommendations for “research and surveillance practices”. Please see article for further details]
- 7.1 Gene therapy?
- Aldén et al. (2022)
- While the CDC claims that the mRNA “vaccines” never enter the nucleus (see Figure 1 and CDC website), a new in vitro study indicates otherwise. The study reports that the results indicate “…a fast up-take of BNT162b2 [Pfizer/BioNTech] into human liver cell line Huh7, leading to changes in LINE-1 expression and distribution. We also show that BNT162b2 mRNA is reverse transcribed intracellularly into DNA in as fast as 6 h upon BNT162b2 exposure.”
- The authors also indicate the necessity for further investigation in regard to the potential relationship to an autoimmune response. They state that “It is worth investigating if the liver cells also present the vaccine-derived SARS-CoV-2 spike protein, which could potentially make the liver cells targets for previously primed spike protein reactive cytotoxic T cells. There has been case reports on individuals who developed autoimmune hepatitis (Bril et al., 2021) after BNT162b2 vaccination.”
- While the CDC claims that the mRNA “vaccines” never enter the nucleus (see Figure 1 and CDC website), a new in vitro study indicates otherwise. The study reports that the results indicate “…a fast up-take of BNT162b2 [Pfizer/BioNTech] into human liver cell line Huh7, leading to changes in LINE-1 expression and distribution. We also show that BNT162b2 mRNA is reverse transcribed intracellularly into DNA in as fast as 6 h upon BNT162b2 exposure.”
- Aldén et al. (2022)

Figure 3: Screen capture from https://www.cdc.gov/coronavirus/2019-ncov/vaccines/facts.html#print (accessed 03/01/2022)
- “There’s some, ultimately the mRNA vaccines are an example for that cellular gene therapy. I always like to say if we had surveyed two years ago, in the public, would you be willing to take gene or cell therapy and inject it into your body, we would have probably had a 95% refusal rate.” Stefan Oelrich, member of the Board of Management of Bayer and head of the Pharmaceuticals Division, The World Health Summit, October 2021.
- U.S. Food and Drug Administration (2020)
- Table 1, Pg14: lists RNA among other “Commonly Used Gene Therapy Products/Vectors”
- “Human gene therapy: Human gene therapy seeks to modify or manipulate the expression of a gene or to alter the biological properties of living cells for therapeutic use.” Pg28
- Interpretation: by these standards the mRNA vaccines ARE gene therapy
- Table 1, Pg14: lists RNA among other “Commonly Used Gene Therapy Products/Vectors”
- 7.2 Failure to properly investigate the vaccines
- “Revelations of poor practices at a contract research company helping to carry out Pfizer’s pivotal covid-19 vaccine trial raise questions about data integrity and regulatory oversight.” (Thacker, 2021)
- “None of the trials currently under way are designed to detect a reduction in any serious outcome such as hospital admissions, use of intensive care, or deaths. Nor are the vaccines being studied to determine whether they can interrupt transmission of the virus” (Doshi, 2020; 2021)
- Additionally, see Kostoff et al., (2021) under “Children, adolescents and vaccinations” below.
- “Revelations of poor practices at a contract research company helping to carry out Pfizer’s pivotal covid-19 vaccine trial raise questions about data integrity and regulatory oversight.” (Thacker, 2021)
- 7.3 Lack of transparency, potential conflicts of interest, corruption
- There are numerous situations that warrant consideration of corruption. The following highlight some of the issues/realities society is facing:
- The FDA’s intentional dragging of the release of documents/data submitted by Pfizer to the public till 2076 (500 pages per month)– data/information that directly pertains to matters that directly impact public health (see Public Health and Medical Professionals for Transparency vs Food and Drug Administration, 11/15/21). The courts, however, have now ordered that “The FDA shall produce the “more than 12,000 pages” articulated in its own proposal, see ECF No. 29 at 24, on or before January 31, 2022.” And “…shall produce the remaining documents at a rate of 55,000 pages every 30 days, with the first production being due on or before March 1, 2022, until production is complete.” (see Public Health and Medical Professionals for Transparency vs Food and Drug Administration, 01/06/22).
- Doshi et al. (2022):
- Referring to the influenza pandemic of 2009 (swine flu pandemic caused by H1N1), the authors address how it was revealed that “…governments around the world had spent billions stockpiling antivirals for influenza that had not been shown to reduce the risk of complications, hospital admissions, or death. The majority of trials that underpinned regulatory approval and government stockpiling of oseltamivir (Tamiflu) were sponsored by the manufacturer; most were unpublished, those that were published were ghostwritten by writers paid by the manufacturer, the people listed as principal authors lacked access to the raw data, and academics who requested access to the data for independent analysis were denied (Cohen, 2009; Doshi, 2009; Godlee, 2009; Godlee and Clarke, 2009).”
- The authors then continue by stating that “The errors are being repeated. Memories are short. Today, despite the global rollout of covid-19 vaccines and treatments, the anonymised participant level data underlying the trials for these new products remain inaccessible to doctors, researchers, and the public—and are likely to remain that way for years to come (El Sahly et al., 2021). This is morally indefensible for all trials, but especially for those involving major public health interventions”
- Referring to the influenza pandemic of 2009 (swine flu pandemic caused by H1N1), the authors address how it was revealed that “…governments around the world had spent billions stockpiling antivirals for influenza that had not been shown to reduce the risk of complications, hospital admissions, or death. The majority of trials that underpinned regulatory approval and government stockpiling of oseltamivir (Tamiflu) were sponsored by the manufacturer; most were unpublished, those that were published were ghostwritten by writers paid by the manufacturer, the people listed as principal authors lacked access to the raw data, and academics who requested access to the data for independent analysis were denied (Cohen, 2009; Doshi, 2009; Godlee, 2009; Godlee and Clarke, 2009).”
- Tanveer et al. (2021): in relation to the necessity for data transparency –
- “Tax payers helped fund COVID-19 vaccine trials and should have the right to access the results.
- There is inadequate availability of COVID-19 vaccine trial documents and data; individual [deidentified] participant data will not be available for months, perhaps years, for most vaccines.
- Widespread use of interventions without full data transparency raises concerns over the rational use of COVID-19 vaccines.”
- “Tax payers helped fund COVID-19 vaccine trials and should have the right to access the results.
- The FDA’s intentional dragging of the release of documents/data submitted by Pfizer to the public till 2076 (500 pages per month)– data/information that directly pertains to matters that directly impact public health (see Public Health and Medical Professionals for Transparency vs Food and Drug Administration, 11/15/21). The courts, however, have now ordered that “The FDA shall produce the “more than 12,000 pages” articulated in its own proposal, see ECF No. 29 at 24, on or before January 31, 2022.” And “…shall produce the remaining documents at a rate of 55,000 pages every 30 days, with the first production being due on or before March 1, 2022, until production is complete.” (see Public Health and Medical Professionals for Transparency vs Food and Drug Administration, 01/06/22).
- There are numerous situations that warrant consideration of corruption. The following highlight some of the issues/realities society is facing:
- 7.4 Potential spread of “Vaccine” mRNA (and side effects) beyond site of injection and to others
- Despite the initial insistence that the vaccine would not spread beyond the site of injection, and accusations of those who stated such as being conspiracy theorists, the evidence continues to accumulate indicating that it does.
- “We found that vaccine-associated synthetic mRNA persists in systemic circulation for at least 2 weeks.” (Fertig et al., 2022)
- “These data demonstrate for the first time to our knowledge the biodistribution of COVID-19 vaccine mRNA to mammary cells and the potential ability of tissue EVs [Extracellular vesicles] to package the vaccine mRNA that can be transported to distant cells. Little has been reported on lipid nanoparticle biodistribution and localization in human tissues after COVID-19 mRNA vaccination. In rats, up to 3 days following intramuscular administration, low vaccine mRNA levels were detected in the heart, lung, testis, and brain tissues, indicating tissue biodistribution.4 We speculate that, following the vaccine administration, lipid nanoparticles containing the vaccine mRNA are carried to mammary glands via hematogenous and/or lymphatic routes.5,6 Furthermore, we speculate that vaccine mRNA released into mammary cell cytosol can be recruited into developing EVs that are later secreted in EBM [expressed breast milk].” (Hanna et al., 2022)
- “Vaccine mRNA-carrying lipid nanoparticles spread after injection throughout the body according to available animal studies and vaccine mRNA (naked or in nanoparticles or in natural exosomes) is found in the bloodstream as well as vaccine spike in free form or encapsulated in exosomes (shown in human studies). Lipid nanoparticles (or their natural equivalent, exosomes or extracellular vesicles (EVs)) have been shown to be able to be excreted through body fluids (sweat, sputum, breast milk) and to pass the transplacental barrier. These EVs are also able to penetrate by inhalation and through the skin (healthy or injured) as well as orally through breast milk (and why not during sexual intercourse through semen, as this has not been studied).” (Banoun, 2022)
- “We found that vaccine-associated synthetic mRNA persists in systemic circulation for at least 2 weeks.” (Fertig et al., 2022)
- Despite the initial insistence that the vaccine would not spread beyond the site of injection, and accusations of those who stated such as being conspiracy theorists, the evidence continues to accumulate indicating that it does.
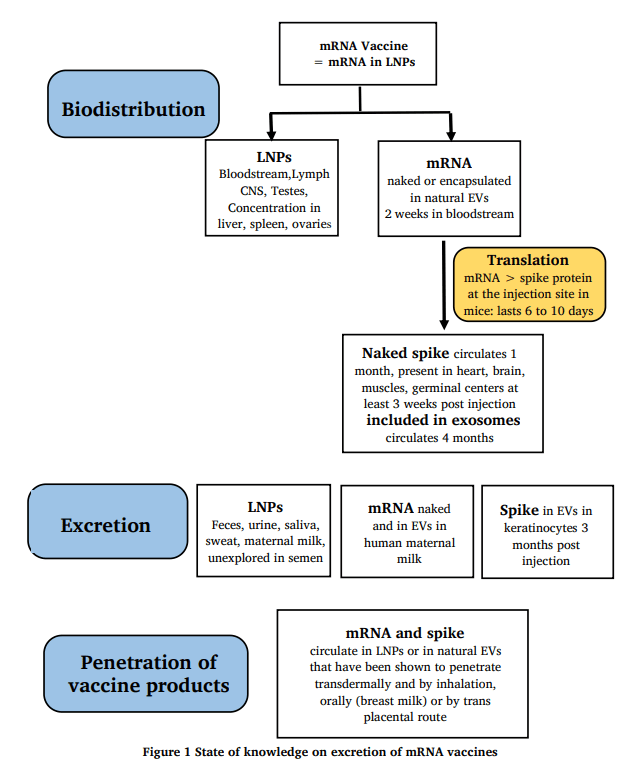
Figure 4: Figure 1 from Banoun, H. (2022). Current state of knowledge on the excretion of mRNA and spike produced by anti-COVID-19 mRNA vaccines; possibility of contamination of the entourage of those vaccinated by these products. Infectious Diseases Research 3.
- In a News & Analysis item in the Journal of the American Medical Association (JAMA), Rita Rubin addresses the potential and current investigation for the administration of the COVID vaccines via an intranasal spray (Rubin, 2021). [NOTE: The article includes comments from an interview with various scientist having potential significant conflicts of interest (see paper for Conflict of Interest Disclosure). The report also reports Dr. Paul Spearman indicating that “One concern with intranasal vaccines is that they could trigger respiratory illnesses”. Given:
- The availability of medications with potentially significantly lower risk of causing the significant adverse events observed with the COVID-19 “vaccines”, such as hypertonic saline and Povidone-Iodine oronasal sprays (see under Available Treatments below), in addition to the available treatments,
- The volume of adverse events reported thus far from the vaccines,
- The potential long-term negative effects of these vaccines being unknown,
- The low infection fatality rate of the diseases (COVID-19) itself and
- The increased potential to enhance the spread to the brain (including due to of the high velocity of the sprayed droplets) – This is particularly disturbing for several reasons:
- The potential for neurological issues is already a concern with the vaccines (Merchant, 2021)
- “SARS-CoV-2 [i.e., the virus itself] has been reported to show a capacity for invading the brains of humans and model animals” (Zhang et al., 2021c)
- “We demonstrate SARS-CoV-2 can infect and replicate in human iPSC-derived neurons and that infection shows limited anti-viral and inflammatory responses but increased activation of EIF2 signaling following infection…Collectively, these findings contribute to previous work demonstrating the ability of SARS-CoV-2 to infect neurons…” (Olivarria et al., 2021)
- “SARS-CoV-2-infected mice exhibited encephalitis hallmarks characterized by production of cytokines and chemokines, leukocyte infiltration, hemorrhage and neuronal cell death. SARS-CoV-2 was also found to productively infect cells within the nasal turbinate, eye and olfactory bulb, suggesting SARS-CoV-2 entry into the brain by this route after intranasal infection. Our data indicate that direct infection of CNS cells together with the induced inflammatory response in the brain resulted in the severe disease observed in SARS-CoV-2-infected K18-hACE2 mice.” (Kumari et al., 2021)
- Easier access to the brain by application of pharmaceuticals to the nares through a route known as nose-to-brain is an established concept: “One way to bypass the blood brain barrier and thus treat diseases of the brain is to use the nasal route of administration and deposit drugs at the olfactory region of the nares, from where they travel to the brain via mechanisms that are still not clearly understood, with travel across nerve fibers and travel via a perivascular pathway both being hypothesized. The nose-to-brain route has been demonstrated repeatedly in preclinical models, with both solution and particulate formulations.” (Wang et al., 2019)
it does not appear that the benefits outweigh the risks, or that this form of “vaccination” would be anymore justifiable than the injectable vaccines.]
- The potential for neurological issues is already a concern with the vaccines (Merchant, 2021)
- The availability of medications with potentially significantly lower risk of causing the significant adverse events observed with the COVID-19 “vaccines”, such as hypertonic saline and Povidone-Iodine oronasal sprays (see under Available Treatments below), in addition to the available treatments,
- Suzuki and Gychka (2021)
- “However, recent observations suggest that the SARS-CoV-2 spike protein can by itself trigger cell signaling that can lead to various biological processes. It is reasonable to assume that such events, in some cases, result in the pathogenesis of certain diseases.”
- “However, we need to consider their long-term consequences carefully, especially when they are administered to otherwise healthy individuals as well as young adults and children. In addition to evaluating data that will become available from SARS-CoV-2 infected individuals”
- “However, recent observations suggest that the SARS-CoV-2 spike protein can by itself trigger cell signaling that can lead to various biological processes. It is reasonable to assume that such events, in some cases, result in the pathogenesis of certain diseases.”
- Appropriate biodistribution studies appear to be, in general, absent from the scientific literature.
- Unfounded insistence (given conflicting evidence) that the vaccine acts locally at the site of injection in order to induce the immune response, despite evidence that this, potentially, is not the case (Pfizer;European Medicines Agency Committee for Medicinal Products for Human Use (CHMP), 2021a;b).
- Tissue implicated in this distribution include the spleen and ovaries after 48 hours (Pfizer). If the particles containing the instructions (mRNA) for making the protein that causes the immune response travel beyond the site of injection, given the target (Kuhn et al., 2004;Hoffmann et al., 2020;Lan et al., 2020) and its broad distribution (Hamming et al., 2004;Jing et al., 2020) in the human body (including the ovaries, brain etc.), this may potentially be the reason for the significantly higher levels of various serious side effects compared to other vaccines (data from VAERS available upon request, and see below)
- Potential toxicity of nanoparticles used in drug delivery
- Wang et al. (2018)
1) “Previous studies have shown that numerous types of NPs [nanoparticles] are able to pass certain biological barriers and exert toxic effects on crucial organs, such as the brain, liver, and kidney.”
2) “NPs can pass through the blood–testis barrier, placental barrier, and epithelial barrier, which protect reproductive tissues, and then accumulate in reproductive organs.”
3) “Previous studies have shown that NPs [nanoparticles] can increase inflammation, oxidative stress, and apoptosis and induce ROS, causing damage at the molecular and genetic levels which results in cytotoxicity.” - McAuliffe and Perry (2009)
1) “While research into the potential reproductive toxicity of nanoparticles is still in its infancy, the identified research suggests that nanoparticles cross the blood testes barrier and deposit in the testes, and that there is potential for adverse effects on sperm cells.”
- Wang et al. (2018)
- Khayat-Khoei et al. (2021)
- “Our observations suggest that, in some individuals, COVID-19 vaccination [Moderna (n = 3) or Pfizer (n = 4)] may carry a short-term risk of CNS demyelination.” [NOTE: study carried out in MS patients; very small number; additional research is necessary]
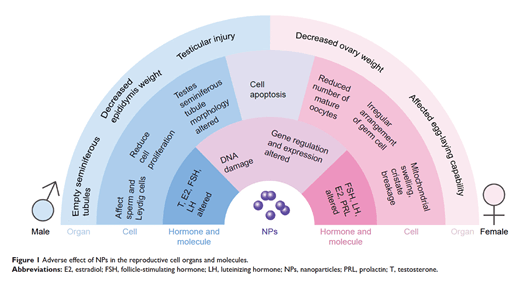
Figure 5: Figure 1 from Wang, R., Song, B., Wu, J., Zhang, Y., Chen, A., and Shao, L. (2018). Potential adverse effects of nanoparticles on the reproductive system. Int J Nanomedicine 13, 8487-8506.
- 7.5 Children, adolescents and vaccinations – lack of necessity and dangers
- Eberhardt and Siegrist (2021)
- “Compared to adults, the incidence and disease severity of COVID-19 are low in children, and despite their infectiveness, their role in disease propagation is limited. Therefore, COVID-19 vaccines will need to have fully demonstrated safety and efficacy in preventing not only complications but transmission to justify childhood vaccination.”
- “To date, no NHP preclinical study has assessed the effect of vaccination in the prevention of transmission, and end-points of human COVID-19 vaccine trials focus on the induction of immunity and individual protection against disease.”
- “Compared to adults, the incidence and disease severity of COVID-19 are low in children, and despite their infectiveness, their role in disease propagation is limited. Therefore, COVID-19 vaccines will need to have fully demonstrated safety and efficacy in preventing not only complications but transmission to justify childhood vaccination.”
- Opel et al. (2021)
- “The criterion that should be prioritized over all others is the first: there must be evidence that a COVID-19 vaccine is safe for children with an acceptable level of risk. Fulfilling this criterion normally requires both prelicensure safety data and data from post licensure studies to monitor for adverse effects after the vaccine has been administered to many people. Accumulation of the data needed to fulfill this criterion often requires years of research.”
- “It would be a mistake to consider making a COVID-19 vaccine mandatory without these data. Vaccine safety is fundamental to maintaining the public’s trust in vaccines, and skirting this safety criterion could have far-reaching consequences”
- “Nevertheless, with these criteria as a framework, the only logical conclusion is that we currently know too little about the performance of any of the candidate COVID-19 vaccines or the epidemiology of SARS-CoV-2 in children to make any firm judgments about whether a COVID-19 vaccine should be mandatory in children.”
- “The criterion that should be prioritized over all others is the first: there must be evidence that a COVID-19 vaccine is safe for children with an acceptable level of risk. Fulfilling this criterion normally requires both prelicensure safety data and data from post licensure studies to monitor for adverse effects after the vaccine has been administered to many people. Accumulation of the data needed to fulfill this criterion often requires years of research.”
- Kostoff et al. (2021)
- “Clinical trials for these inoculations were very short-term (a few months), had samples not representative of the total population, and for adolescents/children, had poor predictive power because of their small size.”
- “Further, the clinical trials did not address changes in biomarkers that could serve as early warning indicators of elevated predisposition to serious diseases.”
- “Most importantly, the clinical trials did not address long-term effects that, if serious, would be borne by children/adolescents for potentially decades.”
- “…the deaths following inoculation are not coincidental and are strongly related to inoculation through strong clustering around the time of injection.”
- “…the VAERS deaths reported so far are for the very short term. We have no idea what the death numbers will be in the intermediate and long-term; the clinical trials did not test for those.”
- “The clinical trials used a non-representative younger and healthier sample to get EUA for the injection. Following EUA, the mass inoculations were administered to the very sick (and first responders) initially, and many died quite rapidly. However, because the elderly who died following COVID-19 inoculation were very frail with multiple comorbidities, their deaths could easily be attributed to causes other than the injection (as should have been the case for COVID-19 deaths as well).”
- “Since many of these potential serious adverse effects have built-in lag times of at least six months or more, we won’t know what they are until most of the population has been inoculated, and corrective action may be too late”
- “Clinical trials for these inoculations were very short-term (a few months), had samples not representative of the total population, and for adolescents/children, had poor predictive power because of their small size.”
- Schauer et al. (2021a)
- “We describe 13 patients 12-17 years of age who presented with chest pain within 1 week after the second dose of the Pfizer vaccine and were found to have elevated serum troponin levels and evidence of myopericarditis.”
- “Although a causal relationship between vaccine receipt and development of myopericarditis cannot be concluded from a case series, clustering in time as well as the uncommon occurrence of myopericarditis and the rapid resolution of symptoms and findings made this likely to be a unique vaccine related event.”
- “Identification of myopericarditis as an adverse event should have high priority during investigations before and after authorization of COVID-19 vaccines and be considered by policy makers in the risk/benefit ratio in adolescents and children”
- “We describe 13 patients 12-17 years of age who presented with chest pain within 1 week after the second dose of the Pfizer vaccine and were found to have elevated serum troponin levels and evidence of myopericarditis.”
- See also VAERS data analysis below
- Eberhardt and Siegrist (2021)
- 7.6 Antibody-Dependent Enhancement (ADE)
- Definition: “ADE is an enhancement of viral entry into immune cells mediated by antibody” (Wu et al., 2020)
- NOTE: Potential reason for ADE, in addition to the dangers of full-length spike-based vaccines – “Our findings provide evidence of the spike protein hijacking the DNA damage repair machinery and adaptive immune machinery in vitro. We propose a potential mechanism by which spike proteins may impair adaptive immunity by inhibiting DNA damage repair…which is also consistent with a recent study that a full–length spike–based vaccine induced lower antibody titers compared to the RBD [receptor-binding-domain]–based vaccine” (Jiang and Mei, 2021).
- “Prior COVID-19 infection but not ongoing Long-COVID [Long-COVID was defined as symptoms persisting >2 months to vaccination] symptoms were associated with an increase in the risk of self-reported adverse events following BNT162b2/Pfizer vaccination.” (Raw et al., 2021) [NOTE: this finding appears to indicate that vaccination (at the very least with this vaccine) is contraindicated following infection]
- “Our results revealed that ADE mediated by SARS-CoV-2 spike-specific antibodies could result from binding to the receptor in slightly different pattern from antibodies mediating neutralizations.” (Wu et al., 2020)
- “Data from the study of SARS-CoV and other respiratory viruses suggest that anti-SARS-CoV-2 antibodies could exacerbate COVID-19 through antibody-dependent enhancement (ADE).” (Lee et al., 2020)
- “Antibody-dependent enhancement (ADE) may be involved in the clinical observation of increased severity of symptoms associated with early high levels of SARS-CoV-2 antibodies in patients. Infants with multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 may also have ADE caused by maternally acquired SARS-CoV-2 antibodies bound to mast cells.” (Ricke, 2021)
- “COVID-19 vaccines designed to elicit neutralizing antibodies may sensitise vaccine recipients to more severe disease than if they were not vaccinated… The specific and significant COVID-19 risk of ADE should have been and should be prominently and independently disclosed to research subjects currently in vaccine trials, as well as those being recruited for the trials and future patients after vaccine approval, in order to meet the medical ethics standard of patient comprehension for informed consent.” (Cardozo and Veazey, 2021)
- In relation to the attempted development for a SARS-CoV-1 vaccine (Tseng et al., 2012):
- “These SARS-CoV vaccines all induced antibody and protection against infection with SARS-CoV. However, challenge of mice given any of the vaccines led to occurrence of Th2-type immunopathology suggesting hypersensitivity to SARS-CoV components was induced. Caution in proceeding to application of a SARS-CoV vaccine in humans is indicated.”
- “…concern for an inappropriate response among persons vaccinated with a SARS-CoV vaccine emanated from experiences with coronavirus infections and disease in animals that included enhanced disease among infected animals vaccinated earlier with a coronavirus vaccine (Perlman and Dandekar, 2005).”
- “The concern arising from the present report is for an immunopathologic reaction occurring among vaccinated individuals on exposure to infectious SARS-CoV, the basis for developing a vaccine for SARS. Additional safety concerns relate to effectiveness and safety against antigenic variants of SARS-CoV and for safety of vaccinated persons exposed to other coronaviruses…”
- “These SARS-CoV vaccines all induced antibody and protection against infection with SARS-CoV. However, challenge of mice given any of the vaccines led to occurrence of Th2-type immunopathology suggesting hypersensitivity to SARS-CoV components was induced. Caution in proceeding to application of a SARS-CoV vaccine in humans is indicated.”
- Definition: “ADE is an enhancement of viral entry into immune cells mediated by antibody” (Wu et al., 2020)
- 7.7 Breakthrough Infections, Vaccine Pressure, Viral Escape
- Background:
- While claims are made that the unvaccinated are a threat to the vaccinated (Goldman, 2021), Kampf (2021a) states that “There is increasing evidence that vaccinated individuals continue to have a relevant role in transmission” the author continues…
- “In the USA, a total of 10 262 COVID-19 cases were reported in vaccinated people by April 30, 2021, of whom 2725 (26·6%) were asymptomatic, 995 (9·7%) were hospitalised, and 160 (1·6%) died (CDC Covid-Vaccine Breakthrough Case Investigations Team, 2021).”
- “In Germany, 55·4% of symptomatic COVID-19 cases in patients aged 60 years or older were in fully vaccinated individuals…”
- “In the USA, a total of 10 262 COVID-19 cases were reported in vaccinated people by April 30, 2021, of whom 2725 (26·6%) were asymptomatic, 995 (9·7%) were hospitalised, and 160 (1·6%) died (CDC Covid-Vaccine Breakthrough Case Investigations Team, 2021).”
- RNA viruses “typically have high mutation rates due to lack of RdRp [RNA-dependent RNA-polymerase or RNA replicase] proofreading activity [i.e. lack of proofreading of the replicated RNA], which promotes viral genetic diversity and increases their adaptive potential.” “…the mutation rates of coronaviruses are an order of magnitude lower (10-6 to10-7) than that of most RNA viruses” (Hartenian et al., 2020)
- “The genomes of positive-strand RNA viruses have considerable capacity to evolve quickly in response to changing ecologic conditions and/or host environments” (Denison et al., 2011)
- While claims are made that the unvaccinated are a threat to the vaccinated (Goldman, 2021), Kampf (2021a) states that “There is increasing evidence that vaccinated individuals continue to have a relevant role in transmission” the author continues…
- Vaccine pressure and viral escape: known to happen with influenza virus due to an “increase of the viral genetic diversity”, which “may reflect the emergence and the subsequent selection of mutants escaping vaccine pressure…”. Admittedly, viral escape with influenza is more likely “particularly where vaccination was not completely or properly applied…” (Cattoli et al., 2011). This raises the following practical and ethical issues:
- Low mortality rate does not justify vaccination
- Low mortality rate does not logically justify the risk of the potential for more dangerous variants that could be more detrimental to the population due to vaccine pressure
- Low mortality rate does not ethically justify the imposition of vaccine mandates simply to reach a “complete vaccination state”, when natural immunity could continue to evolve and effective medications for treatment are available.
- Summary: While coronaviruses are somewhat more stable, they still have the capacity to mutate. This is evident in the SARS-CoV-2 variants that continue to appear (e.g. Delta variant). Vaccine breakthrough appears to be indicated given the higher presence in those vaccinated. Weighing the risk of death from the virus with the purported benefits of the vaccine, is it practical and ethical to provide a vaccine that may be more likely to produce a more dangerous situation? [also see below Saito et al., (2021)]
- Low mortality rate does not justify vaccination
- “The proportion with breakthrough infections was 3 times higher in the IC [immunocompromised] cohort compared to the non-IC cohort” (Di Fusco et al., 2021) (NOTE: Asthma is also considered as an immunocompromised stated (Christou et al., 2019). However, the authors indicate that only the following were considered in the immunocompromised group (IC): “(1) symptomatic human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS); (2) solid malignancy; (3) bone marrow transplant; (4) organ transplant (excluding bone marrow transplant); (5) rheumatologic or other inflammatory condition; (6) a primary immunodeficiency; (7) other immune conditions; (8) chronic kidney disease (CKD) or end stage renal disease (ESRD); and (9) hematologic malignancy.”
- “Outbreak investigations suggest that vaccinated persons can spread Delta” (Riemersma et al., 2021)
- “Approximately three quarters (346; 74%) of cases occurred in fully vaccinated persons (those who had completed a 2-dose course of mRNA vaccine [Pfizer-BioNTech or Moderna] or had received a single dose of Janssen [Johnson & Johnson] vaccine ≥14 days before exposure).” (Brown et al., 2021)
- “Our data show that anti-disease vaccines that do not prevent transmission can create conditions that promote the emergence of pathogen strains that cause more severe disease in unvaccinated hosts.” (Read et al., 2015)
- The combined findings from
- Riemersma et al. (2021), indicating that vaccinated people can spread the Delta (also known as B.1.617.2) variant,
- Read et al. (2015), indicating that vaccines that do not prevent transmission can promote the emergence of strains that can cause more severe disease, and
- Saito et al. (2021) who report a specific mutation (P681R) in the spike protein characteristic of the Delta/ B.1.617.2 variant which facilitates “the spike protein cleavage and enhances viral fusogenicity” and that viruses that have this mutation exhibit “higher pathogenicity than the parental virus”,
appear to give further credence to the concept of viral escape.
- Riemersma et al. (2021), indicating that vaccinated people can spread the Delta (also known as B.1.617.2) variant,
- Background:
- 7.8 Virus interference
- “Receiving influenza vaccination may increase the risk of other respiratory viruses, a phenomenon known as virus interference…Vaccine derived virus interference was significantly associated with coronavirus and human metapneumovirus…” (Wolff, 2020)
- 7.9 Measuring Vaccine Efficacy
- What is the real efficacy of the vaccines? – It depends on what you’re looking at!!!! Olliaro et al. (2021) present some useful information in this regard:
- Relative Risk Reduction (RRR)
- Definition: used to report vaccine efficacy = 1 – RR (the ratio of attack rates with and without a vaccine).
- “However, RRR should be seen against the background risk of being infected and becoming ill with COVID-19, which varies between populations and over time.”
- “RRR considers only participants who could benefit from the vaccine”
- The RRR for the vaccines is:
- 95% for the Pfizer–BioNTech,
- 94% for the Moderna–NIH,
- 91% for the Gamaleya,
- 67% for the J&J, and
- 67% for the AstraZeneca–Oxford vaccines
- Definition: used to report vaccine efficacy = 1 – RR (the ratio of attack rates with and without a vaccine).
- Absolute Risk Reduction (ARR)
- Definition: “the difference between attack rates with and without a vaccine, considers the whole population” (compare to RRR)
- “ARRs tend to be ignored because they give a much less impressive effect size than RRRs”.
- The ARR for the vaccines is:
- 1.3% for the AstraZeneca–Oxford,
- 1.2% for the Moderna–NIH,
- 1.2% for the J&J,
- 0.93% for the Gamaleya, and
- 0.84% for the Pfizer–BioNTech vaccines.
- Definition: “the difference between attack rates with and without a vaccine, considers the whole population” (compare to RRR)
- “There are many lessons to learn from the way studies are conducted and results are presented. With the use of only RRRs, and omitting ARRs, reporting bias is introduced, which affects the interpretation of vaccine efficacy”
- Relative Risk Reduction (RRR)
- What is the real efficacy of the vaccines? – It depends on what you’re looking at!!!! Olliaro et al. (2021) present some useful information in this regard:
- 7.10 Vaccine Efficacy/Inefficacy, Waning Vaccine Immunity and Natural Immunity
- Recent analysis of the CDC data by researchers at the Kaiser Family Foundation indicates the following:
- Deaths of the unvaccinated decreased over time stabilizing at around April, 2022
- Deaths of those vaccinated with primary series remains stable between September 2021 and August 2022
- Deaths of the vaccinated with booster increased from 0.4% (September 2021) to 36% (August 2022), stabilizing around April, 2022
- Combined Deaths of vaccinated with primary series and booster indicates an increase in deaths from COVID-19 vaccination status from 22.4% (September 2021) to 58% (August 2022)
- Deaths of the unvaccinated decreased over time stabilizing at around April, 2022
- Recent analysis of the CDC data by researchers at the Kaiser Family Foundation indicates the following:
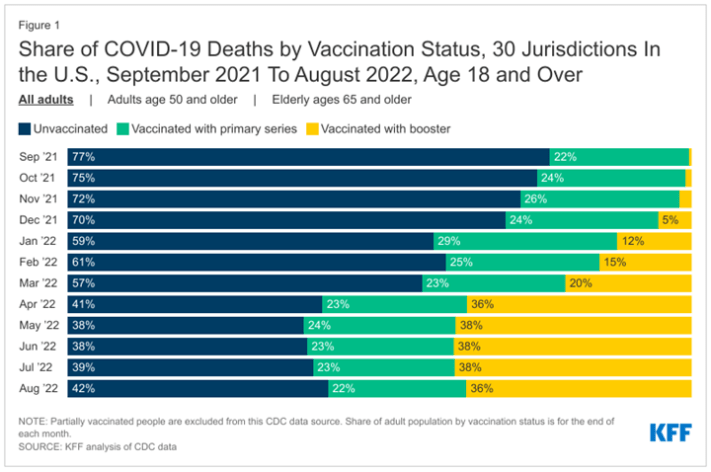
Figure 6: Source: https://www.kff.org/policy-watch/why-do-vaccinated-people-represent-most-covid-19-deaths-right-now/
- In reference to the Fourth BNT162b2 Vaccine Dose: “Time-specific vaccine effectiveness (which, in our analysis, compared infection rates among participants who had not yet been infected since vaccination) waned with time, decreasing from 52% (95% CI, 45 to 58) during the first 5 weeks after vaccination to −2% (95% CI, −27 to 17) at 15 to 26 weeks.” (Canetti et al., 2022)
- “By analyzing results of more than 460,000 individuals, we show that while recent vaccination reduces Omicron viral load, its effect wanes rapidly. In contrast, a significantly slower waning rate is demonstrated for recovered COVID-19 individuals.” (Woodbridge et al., 2022)
- “No discernable differences in protection against symptomatic BA.1 and BA.2 infection were seen with previous infection, vaccination, and hybrid immunity.” (Altarawneh et al., 2022)
- “Among persons who had been previously infected with SARS-CoV-2 (regardless of whether they had received any dose of vaccine or whether they had received one dose before or after infection), protection against reinfection decreased as the time increased since the last immunity-conferring event; however,this protection was higher than that conferred after the same time had elapsed since receipt of a second dose of vaccine among previously uninfected persons. A single dose of vaccine after infection reinforced protection against reinfection.” (Goldberg et al., 2022)
- Number of cases of SARS-CoV-2 infections per 100,000 person-days (For graphical representation of the following information please see the figure below this information from the paper itself)
- 1) Unvaccinated:
- 10.5 (4 to <6 months from recovery from infection)
- 30.2 (1 year or more from recovery from infection)
- 10.5 (4 to <6 months from recovery from infection)
- 2) Single dose after previous infection:
- 3.7 (<2 months from vaccination)
- 11.6 (at least 6 months from vaccination)
- 3.7 (<2 months from vaccination)
- 3) Two doses:
- 21.1 (<2 months from vaccination)
- 88.9 (at least 6 months from vaccination)
- 21.1 (<2 months from vaccination)
- 1) Unvaccinated:
- Number of cases of SARS-CoV-2 infections per 100,000 person-days (For graphical representation of the following information please see the figure below this information from the paper itself)

Figure 7: “Figure 3. Estimated Covariate-Adjusted Rates of Confirmed Infections per 100,000 Person-Days at Risk.” (Goldberg et al., 2022)
- “Objective: To assess the antibody response in non-immunocompromised adults after two doses of BNT162b2 [vaccine]… Conclusions: The decline of anti-RBD antibodies 3 months after the second dose of BNT162b2 is of concern because it raises the possibility of a short-lived humoral immunity after vaccination.” Of course, the solution provided is “Booster doses of BNT162b2 might be required to maintain high titres of anti-RBD antibodies over time.”!!! (Erice et al., 2022)
- Why are Chinese scientists (four out of the five authors are affiliated with an institution in China) so interested in the “Impact of vaccination on the COVID‑19 pandemic in U.S. states”??? The abstract states that “Herd immunity could be achieved earlier with a faster vaccination pace, lower vaccine hesitancy, and higher vaccine effectiveness…. These findings improve our understanding of the COVID-19 vaccination and can inform future public health policies.” (Chen et al., 2022) [Question: What is the motive of this research? Is it really deserving of such attention in such a high-profile scientific journal?]
- “…an Omicron boost may not provide greater immunity or protection compared to a boost with the current mRNA-1273 vaccine” (Gagne et al., 2022) [Interpretation: Omicron-specific vaccine booster may not be needed]
- Three studies (Accorsi et al., 2022; Johnson et al., 2022; Thompson et al., 2022) were published on the same day by the CDC or CDC-associated investigators reporting that the booster shots were needed against Omicron. The vaccine efficacy reported does not appear to concur with expected consequences of the vaccine reported in other studies e.g., reports of increased spread associated with immune evasion (Lyngse et al., 2021), reduction in neutralization with resulting potential increases in breakthrough infections (Gazit et al., 2021;Kampf, 2021b), reduction in long-term protection (Mizrahi et al., 2021), increased risk of infection by variants (Servellita et al., 2021) and the increased number of cases despite vaccination (Subramanian and Kumar, 2021). Additionally, the message propagated by these papers does not appear to concur with the evident reality of hospitalizations e.g., in Israel.
- “Our findings confirm that the rapid spread of the Omicron VOC [variant of concern] primarily can be ascribed to the immune evasiveness rather than an inherent increase in the basic transmissibility…Our results show that the Omicron VOC is generally 2.7-3.7 times more infectious than the Delta VOC among vaccinated individuals…Surprisingly, we observed no significant difference between the SAR of Omicron versus Delta among unvaccinated individuals” (Lyngse et al., 2021)
- Regarding Omicron variant in those vaccinated: “There was a substantial fall in neutralisation titres in recipients of both AZD1222 [AstraZeneca] and BNT162b2 [Pfizer] primary courses, with evidence of some recipients failing to neutralise at all. This will likely lead to increased breakthrough infections in previously infected or double vaccinated individuals, which could drive a further wave of infection, although there is currently no evidence of increased potential to cause severe disease, hospitalisation or death.” (Dejnirattisai et al., 2021)
- “High COVID-19 vaccination rates were expected to reduce transmission of SARS-CoV-2 in populations by reducing the number of possible sources for transmission and thereby to reduce the burden of COVID-19 disease. Recent data, however, indicate that the epidemiological relevance of COVID-19 vaccinated individuals is increasing…Many decision makers assume that the vaccinated can be excluded as a source of transmission. It appears to be grossly negligent to ignore the vaccinated population as a possible and relevant source of transmission when deciding about public health control measures.” (Kampf, 2021b)
- Why are Chinese scientists (four out of the five authors are affiliated with an institution in China) so interested in the “Impact of vaccination on the COVID‑19 pandemic in U.S. states”??? The abstract states that “Herd immunity could be achieved earlier with a faster vaccination pace, lower vaccine hesitancy, and higher vaccine effectiveness…. These findings improve our understanding of the COVID-19 vaccination and can inform future public health policies.” (Chen et al., 2022) [Question: What is the motive of this research? Is it really deserving of such attention in such a high-profile scientific journal?]
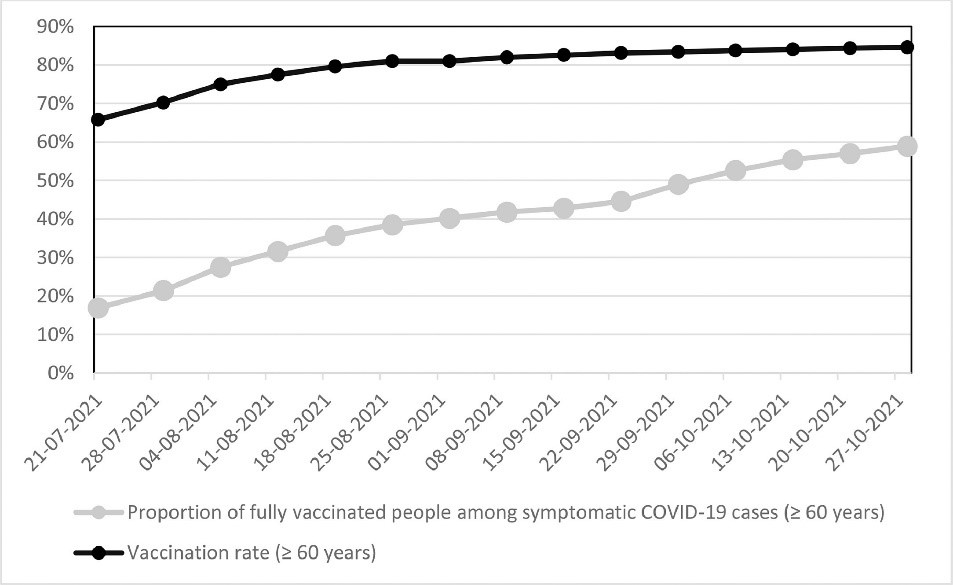
Figure 8: “Figure 1 Vaccination rates and proportions of fully vaccinated people among symptomatic COVID-19 cases (≥ 60 years) in Germany between 21. July and 27. October 2021 based on the weekly reports from the Robert Koch-Institute” (Kampf, 2021b)
- “After controlling for potential confounders as age and comorbidities, we found a significant 1.51 fold (95% CI, 1.38–1.66) increased risk for infection for early vaccinees compared to those vaccinated later that was similar across all ages groups. The increased risk reached 2.26- fold (95% CI, 1.80–3.01) when comparing those who were vaccinated in January to those vaccinated in April. Taken together, the study suggests a possible relative decrease in the long-term protection of the BNT162b2 vaccine against the Delta variant of SARS-CoV-2.” (Mizrahi et al., 2021)
- Report in Veterans Health Administration (n=780,225): “From February to October 2021, VE-I [vaccine effectiveness against infection] declined from 87.9% to 48.1%, and the decline was greatest for the Janssen vaccine resulting in a VE-I of 13.1%…From July to October 2021, VE-D [vaccine effectiveness against death] for age 65 years was 73.0% for Janssen, 81.5% for Moderna, and 84.3% for Pfizer-BioNTech; VE-D for age ≥65 years was 52.2% for Janssen, 75.5% for Moderna, and 70.1% for Pfizer-BioNTech.” (Cohn et al., 2021)
- “The spike protein of SARS-CoV-2 variant A.30 is heavily mutated and evades vaccine-induced antibodies with high efficiency” (Arora et al., 2021)
- “Vaccine effectiveness against symptomatic Covid-19 infection wanes progressively over time across all subgroups, but at different rate according to type of vaccine, and faster for men and older frail individuals. The effectiveness against severe illness seems to remain high through 9 months, although not for men, older frail individuals, and individuals with comorbidities. This strengthens the evidence-based rationale for administration of a third booster dose.” (Nordström et al., 2021) [NOTE: and the fourth booster? And the nth booster?]
- Servellita et al. (2021)
- “Fully vaccinated were more likely than unvaccinated persons to be infected by variants carrying mutations associated with decreased antibody neutralization (L452R, L452Q, E484K, and/or F490S) (78% versus 48%, p = 1.96e-08), but not by those associated with increased infectivity only (N501Y) (85% versus 77%, p = 0.092)” – see Figure below [NOTE: This indicates that fully vaccinated people, in fact more so, can still be infected by the virus]
- “Taken together, these data suggest that symptomatic breakthrough cases are likely as infectious as symptomatic unvaccinated cases, and thus may contribute to ongoing SARS-CoV-2 transmission, even in a highly vaccinated community.”
- “Fully vaccinated were more likely than unvaccinated persons to be infected by variants carrying mutations associated with decreased antibody neutralization (L452R, L452Q, E484K, and/or F490S) (78% versus 48%, p = 1.96e-08), but not by those associated with increased infectivity only (N501Y) (85% versus 77%, p = 0.092)” – see Figure below [NOTE: This indicates that fully vaccinated people, in fact more so, can still be infected by the virus]
Figure 9: From Figure 2B Servellita et al., 2021 “(B) Pie charts showing the proportions of SARS-CoV-2 genomes carrying mutations associated with antibody resistance (top) and increased infectivity (bottom) in fully vaccinated and unvaccinated cases from UCSF Hospitals and Clinics and Color Genomics Laboratory, and partially vaccinated cases from UCSF. Red color indicates the presence of mutations associated with antibody resistance, green indicates the presence of mutations associated with increased infectivity, and black indicates the absence of either mutations.”
- “We found no significant difference in cycle threshold values between vaccinated and unvaccinated, asymptomatic and symptomatic groups infected with SARS-CoV-2 Delta.” (Acharya et al., 2021) [NOTE: this indicates that vaccinated people are just as likely to spread the virus]
- “Six months after receipt of the second dose of the BNT162b2 vaccine, humoral response was substantially decreased, especially among men, among persons 65 years of age or older, and among persons with immunosuppression.”(Levin et al., 2021)
- “BNT162b2-induced protection against SARS-COV-2 infection appeared to wane rapidly [Vaccine Effectiveness: 22.3%] following its peak [Vaccine Effectiveness: 77.5%] after the second dose, but protection against hospitalization and death persisted at a robust level for 6 months after the second dose.” (Chemaitelly et al., 2021)
- Subramanian and Kumar (2021)
- “Notably, Israel with over 60% of their population fully vaccinated had the highest COVID-19 cases per 1 million people in the last 7 days. The lack of a meaningful association between percentage population fully vaccinated and new COVID-19 cases is further exemplified, for instance, by comparison of Iceland and Portugal. Both countries have over 75% of their population fully vaccinated and have more COVID-19 cases per 1 million people than countries such as Vietnam and South Africa.”
- “There also appears to be no significant signaling of COVID-19 cases decreasing with higher percentages of population fully vaccinated”
- “Of the top 5 counties that have the highest percentage of population fully vaccinated (99.9–84.3%), the US Centers for Disease Control and Prevention (CDC) identifies 4 of them as “High” Transmission counties… Conversely, of the 57 counties that have been classified as “low” transmission counties by the CDC, 26.3% (15) have percentage of population fully vaccinated below 20%.”
- “…in a report released from the Ministry of Health in Israel, the effectiveness of 2 doses of the BNT162b2 (Pfizer-BioNTech) vaccine against preventing COVID-19 infection was reported to be 39% [6], substantially lower than the trial efficacy of 96% [7].”
- “Even though vaccinations offers protection to individuals against severe hospitalization and death, the CDC reported an increase from 0.01 to 9% and 0 to 15.1% (between January to May 2021) in the rates of hospitalizations and deaths, respectively, amongst the fully vaccinated [10]”.
- “Notably, Israel with over 60% of their population fully vaccinated had the highest COVID-19 cases per 1 million people in the last 7 days. The lack of a meaningful association between percentage population fully vaccinated and new COVID-19 cases is further exemplified, for instance, by comparison of Iceland and Portugal. Both countries have over 75% of their population fully vaccinated and have more COVID-19 cases per 1 million people than countries such as Vietnam and South Africa.”
- Gazit et al. (2021)
- “SARS-CoV-2-naïve vaccinees [not previously infected, received vaccine] had a 13.06-fold (95% CI, 8.08 to 21.11) increased risk for breakthrough infection with the Delta variant compared to those previously infected, when the first event (infection or vaccination) occurred during January and February of 2021.”
- “When allowing the infection to occur at any time before vaccination (from March 2020 to February 2021), evidence of waning natural immunity was demonstrated, though SARS-CoV-2 naïve vaccinees [not previously infected, received vaccine] had a 5.96-fold (95% CI, 4.85 to 7.33) increased risk for breakthrough infection and a 7.13-fold (95% CI, 5.51 to 9.21) increased risk for symptomatic disease. SARS-CoV-2-naïve vaccinees were also at a greater risk for COVID-19-related-hospitalizations compared to those that were previously infected.”
- “SARS-CoV-2-naïve vaccinees [not previously infected, received vaccine] had a 13.06-fold (95% CI, 8.08 to 21.11) increased risk for breakthrough infection with the Delta variant compared to those previously infected, when the first event (infection or vaccination) occurred during January and February of 2021.”
- “Efficacy peaked at 96.2% during the interval from 7 days to <2 months post-dose 2, and declined gradually to 83.7% from 4 months post-dose 2 to the data cut-off, an average decline of ~6% every 2 months.” (Thomas et al., 2021)
- Vaccine effectiveness between March and July, 2021 significantly reduced from 93.9% to 65.5% (March: 93.9%; April: 96.2%; May: 95.9%; June: 94.3%; July: 65.5%) (Keehner et al., 2021)
- While the vaccines are being pushed as the solution to the COVID-19 pandemic, in addition to the reduced effectiveness just described, even if an argument could be made for their efficacy and reasonable use/administration, there is a factor that is being ignored, makes the use of the vaccines under the current socio-political circumstances even less justifiable – the potential inefficacy of the vaccines because of the various irrational and stresses (addressed above, e.g. lockdowns, mask mandates and more) imposed on whole populations.
- Chronic stress is known to significantly influence physiology (McEwen, 2007;2017;Koob and Schulkin, 2019), including the immune system [needed to combat the virus] (Segerstrom and Miller, 2004; Dhabhar, 2009).
- Chronic stress contributes to the potential for an increased predisposition to psychiatric disorders such as depression and anxiety (Hammen et al., 2009; McEwen et al., 2012).
- Chronic stress also impacts the efficacy of treatments (e.g. psychotropics, vaccinations, etc.) that are used to treat the systems affected by chronic stress itself! This includes central nervous system and the immune system (Dhabhar, 2009; Sommershof et al., 2017; Madison et al., 2021).
- “Psychological and behavioral factors interact with the current pandemic in many ways beyond vaccine response. These factors can influence susceptibility to infection on SARS-CoV-2 exposure and willingness to be vaccinated” (Madison et al., 2021)
- Chronic stress is known to significantly influence physiology (McEwen, 2007;2017;Koob and Schulkin, 2019), including the immune system [needed to combat the virus] (Segerstrom and Miller, 2004; Dhabhar, 2009).
- “This finding, that VoC-RBD-reactive [VoC: variants of concern; RBD:S receptor binding domain] MBCs [memory B-cells] are present in the peripheral blood of all subjects including those that experienced asymptomatic or mild disease, provides a reason for optimism regarding the capacity of vaccination, prior infection, and/or both, to limit disease severity and transmission of variants of concern as they continue to arise and circulate.” (Lyski et al., 2021)
- “SARS-CoV-2 immunity is retained in a significant proportion of mild COVID-19 convalescents 12 months post-infection in the absence of re-exposure to the virus. Despite this, changes in the amino acid sequence of the Spike antigen that are present in current VoC result in virus evasion of neutralising antibodies, as well as evasion of functional T cell responses” (Garcia-Valtanen et al., 2021) [NOTE: The last statement confirms what other research has stated: that virus evasion of the immune system is potentially the result of the actions of the newer variants. What is not addressed is how vaccines may potentially have contributed to this evasion (see other literature in this document)]
- “In this cross-sectional study of unvaccinated US adults, antibodies were detected in 99% of individuals who reported a positive COVID-19 test result, in 55% who believed they had COVID-19 but were never tested, and in 11% who believed they had never had COVID-19 infection. Anti-RBD [SARS-CoV-2 spike protein receptor-binding domain] levels were observed after a positive COVID-19 test result up to 20 months, extending previous 6-month durability data” (Alejo et al., 2022). [NOTE: However, Dr. Paul Alexander makes an important note about this in his blog: “The magic about these studies are that it shows if you ran the study for 100 years you will then conclude that natural immunity lasts 100 years…So its not that natural immunity lasts for 20 months, its the study was stopped then or follow-up ended then.” [sic]]
- “In vaccinated subjects, antibody titers decreased by up to 38% each subsequent month while in convalescents they decreased by less than 5% per month. Six months after BNT162b2 vaccination 16.1% subjects had antibody levels below the seropositivity threshold of <50 AU/mL, while only 10.8% of convalescent patients were below <50 AU/mL threshold after 9 months from SARS-CoV-2 infection. This study demonstrates individuals who received the Pfizer-BioNTech mRNA vaccine have different kinetics of antibody levels compared to patients who had been infected with the SARS-CoV-2 virus, with higher initial levels but a much faster exponential decrease in the first group.” (Israel et al., 2021) [Interpretation: antibody levels were higher at 6 months in “SARS-CoV-2 convalescents who had not received the vaccine” (i.e., in patients who had natural immunity due to a prior COVID-19 infection) relative to those who were vaccinated]
- “Our study provides evidence that the airway immune cells of children are primed for virus sensing, resulting in a stronger early innate antiviral response to SARS-CoV-2 infection than in adults.” (Loske et al., 2021)
- “…that immune protection is robust among those previously infected and that the risk of reinfection is low, and that it is mostly asymptomatic.” (Chvatal-Medina et al., 2021)
- “Reinfections had 90% lower odds of resulting in hospitalization or death than primary infections.” (Abu-Raddad et al., 2021b) – Interpretation: Immunity after infection is protective.
- In a response to a letter from the law firm Siri & Glimstad on behalf of the “Informed Consent Action Network (“ICAN”) under the Freedom of Information Act requesting:
“Documents reflecting any documented case of an individual who: (1) never received a COVID-19 vaccine; (2) was infected with COVID-19 once, recovered, and then later became infected again; and (3) transmitted SARS-CoV-2 to another person when reinfected”
the CDC responded as follows:
“A search of our records failed to reveal any documents pertaining to your request. The CDC Emergency Operations Center (EOC) conveyed that this information is not collected.”
Interpretation: CDC has NO record that those with natural immunity actually transmit COVID-19. (Andoh, 2021; Palmer, 2021) - “Prior SARS-CoV-2 infection was associated with a statistically significantly lower risk [approximately 6 times lower] for breakthrough infection among individuals receiving the BNT162b2 or mRNA-1273 vaccines” (Abu-Raddad et al., 2021)
- NOTE: one of the potential implications of this finding is that the vaccine is actually working against the natural functioning of the immune system. Additionally, the findings support the reports in the previous section addressing the weakened antibody response in those vaccinated relative to those who are not (e.g. see Servellita et al. (2021))
- NOTE: Additionally, these findings appear to corroborate the report by the UK Health Security Agency that mentions “the overall higher profile of antibody levels in those who have experienced past infection is evident” (UK Health Security Agency, 2021)
- NOTE: one of the potential implications of this finding is that the vaccine is actually working against the natural functioning of the immune system. Additionally, the findings support the reports in the previous section addressing the weakened antibody response in those vaccinated relative to those who are not (e.g. see Servellita et al. (2021))
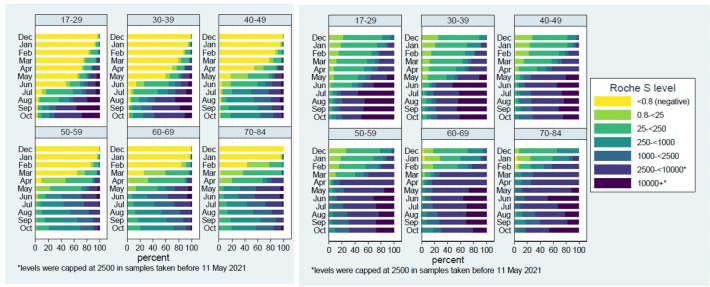
Figure 10: Adapted from (UK Health Security Agency, 2021) Left: “Figure 6: Categorised Roche S antibody levels by age group and month in N negative samples [people who were not previously infected] , December 2020 to October 2021”; Right: “Figure 7: Categorised Roche S antibody levels [people who were previously infected] by age group and month in N positive samples, December 2020 to October 2021”. Interpretation: people who were previously infected appear to have higher antibody levels/stronger immune response (darker colors). However, sadly the authors of the document and the scientists involved appear to still be catching up on what we have known for years in relation to antibody levels: “Researchers across the globe are working to better understand what antibody levels mean in terms of protection against COVID-19. Current thinking is that there is no threshold antibody level that offers complete protection against infection, but instead that higher antibody levels are likely to be associated with lower probability of infection.”!!!
- “The data suggest that immunity in convalescent individuals will be very long lasting and that convalescent individuals who receive available mRNA vaccines will produce antibodies and memory B cells that should be protective against circulating SARS-CoV-2 variants.” [NOTE: the latter part pertaining to the vaccines does not appear to hold given the breakthrough infections, and hospital admissions being observed primarily of people who have received the vaccine] (Wang et al., 2021)
- “Importantly, we detected SARS-CoV-2-reactive CD4+ T cells in ~40%–60% of unexposed individuals, suggesting cross-reactive T cell recognition between circulating ‘‘common cold’’ coronaviruses and SARS-CoV-2.” (Grifoni et al., 2020)
- “Substantial immune memory is generated after COVID-19, involving all four major types of immune memory. About 95% of subjects retained immune memory at ~6 months after infection. Circulating antibody titers were not predictive of T cell memory. Thus, simple serological tests for SARS-CoV-2 antibodies do not reflect the richness and durability of immune memory to SARS-CoV-2. This work expands our understanding of immune memory in humans. These results have implications for protective immunity against SARS-CoV-2 and recurrent COVID-19” (Dan et al., 2021)
- “SARS-CoV-2-specific cellular and humoral immunities are durable at least until one year after disease onset…These findings are encouraging in relation to the longevity of immune memory against this novel virus and indicate that these sustained immune components, which persist, among most SARS-CoV-2-infected individuals, may contribute to protection against reinfection.” (Zhang et al., 2021a)
- “…the results indicate local tissue coordination of cellular and humoral immune memory against SARS-CoV-2 for site-specific protection against future infectious challenges.” (Poon et al., 2021)
- “It is now well-documented that mild and severe infection generates circulating virus-specific T cells and antibodies detectable in peripheral blood for up to a year or more (Grifoni et al., 2020; Bilich et al., 2021; Cohen et al., 2021; Dan et al., 2021; Gaebler et al., 2021; Rodda et al., 2021; Wang et al., 2021; Zuo et al., 2021). Moreover, the presence of neutralizing antibodies specific for the viral Spike (S) protein correlates with protection for SARS-CoV-2 vaccines (Earle et al., 2021; Khoury et al., 2021).” (Poon et al., 2021)
- Gazit et al. (2021)
- “This study demonstrated that natural immunity confers longer lasting and stronger protection against infection, symptomatic disease and hospitalization caused by the Delta variant of SARS-CoV-2, compared to the BNT162b2 two-dose vaccine-induced immunity.”
- Also see same authors under Vaccine In/Effectiveness
- “This study demonstrated that natural immunity confers longer lasting and stronger protection against infection, symptomatic disease and hospitalization caused by the Delta variant of SARS-CoV-2, compared to the BNT162b2 two-dose vaccine-induced immunity.”
- “We found that NAb [neutralizing antibodies] against the wild-type virus persisted in 89% and S-IgG [SARS-CoV-2 spike immunoglobulin G] in 97% of subjects for at least 13 months after infection. Only 36% had N-IgG [nucleoprotein IgG] by 13 months. The mean S-IgG concentrations declined from 8 to 13 months by less than one third; N-IgG concentrations declined by two thirds. Subjects with severe infection had markedly higher IgG and NAb levels and are expected to remain seropositive for longer.” (Haveri et al., 2021)
- “Overall, our results indicate that mild infection with SARS-CoV-2 induces robust antigen-specific, long-lived humoral immune memory in humans.” (Turner et al., 2021)
- “The observation that memory B cell responses do not decay after 6.2 months but instead continue to evolve, is strongly suggestive that individuals who are infected with SARS-CoV-2 could mount a rapid and effective response to the virus upon re-exposure.” (Gaebler et al., 2021)
- “The finding that patients who recovered from COVID-19 and SARS can mount T cell responses against shared viral determinants suggests that previous SARS-CoV infection can induce T cells that are able to cross-react against SARS-CoV-2…These findings demonstrate that virus-specific T cells induced by infection with betacoronaviruses are long-lasting, supporting the notion that patients with COVID-19 will develop long-term T cell immunity. Our findings also raise the possibility that long-lasting T cells generated after infection with related viruses may be able to protect against, or modify the pathology caused by, infection with SARS-CoV-2.” (Le Bert et al., 2020)
- “The study results suggest that reinfections are rare events and patients who have recovered from COVID-19 have a lower risk of reinfection.” (Vitale et al., 2021)
- “Importantly, SARS-CoV-2-specific T cells were detectable in antibody-seronegative exposed family members and convalescent individuals with a history of asymptomatic and mild COVID-19. Our collective dataset shows that SARS-CoV-2 elicits broadly directed and functionally replete memory T cell responses, suggesting that natural exposure or infection may prevent recurrent episodes of severe COVID-19.” (Sekine et al., 2020)
- 7.11 Adverse events/Side effects & VAERS data analysis
- Abstract: “Cases of myocarditis, diagnosed clinically by laboratory tests and imaging have been described in the context of mRNA-based anti-SARS-CoV-2 vaccination…We describe the autopsy findings and common characteristics of myocarditis in untreated persons who received anti-SARS-CoV-2 vaccination. Standardized autopsies were performed on 25 persons who had died unexpectedly and within 20 days after anti-SARS-CoV-2 vaccination. In four patients who received a mRNA vaccination, we identified acute (epi-) myocarditis without detection of another significant disease or health constellation that may have caused an unexpected death. Histology showed patchy interstitial myocardial T-lymphocytic infiltration, predominantly of the CD4 positive subset, associated with mild myocyte damage. Overall, autopsy findings indicated death due to acute arrhythmogenic cardiac failure. Thus, myocarditis can be a potentially lethal complication following mRNA-based anti-SARS-CoV-2 vaccination…” (Schwab et al., 2022)
- Patone et al. (2022)
- “Associations [to a risk of myocarditis] were stronger in men younger than 40 years for all vaccines. In men younger than 40 years old, the number of excess myocarditis events per million people was higher after a second dose of mRNA-1273 than after a positive SARS-CoV-2 test…”
- “However, the risk of myocarditis after vaccination is higher in younger men, particularly after a second dose of the mRNA-1273 vaccine.”
- [Limitations: There are some limitations to this study: The study is limited to 28 days after vaccination; While the study makes statements addressing lower risks than “after a positive SARS-CoV-2 test”, the subjects in the study are described as being “13 years or older vaccinated for COVID-19” i.e. the study does not appear to have a control unvaccinated group, yet it makes assertions that “the risk of hospitalization or death from myocarditis after SARS-CoV-2 infection is substantially higher than the risk associated with a first dose of ChAdOx1, and a first, second, or booster dose of BNT162b2 mRNA vaccine]
- “Associations [to a risk of myocarditis] were stronger in men younger than 40 years for all vaccines. In men younger than 40 years old, the number of excess myocarditis events per million people was higher after a second dose of mRNA-1273 than after a positive SARS-CoV-2 test…”
- Lee et al. (2022)
- “In this sample, 42% of people with regular menstrual cycles bled more heavily than usual, while 44% reported no change after being vaccinated. Among respondents who typically do not menstruate, 71% of people on long-acting reversible contraceptives, 39% of people on gender-affirming hormones, and 66% of postmenopausal people reported breakthrough bleeding. We found that increased/breakthrough bleeding was significantly associated with age, systemic vaccine side effects (fever and/or fatigue), history of pregnancy or birth, and ethnicity. Generally, changes to menstrual bleeding are not uncommon or dangerous, yet attention to these experiences is necessary to build trust in medicine.”
- “In this sample, 42% of people with regular menstrual cycles bled more heavily than usual, while 44% reported no change after being vaccinated. Among respondents who typically do not menstruate, 71% of people on long-acting reversible contraceptives, 39% of people on gender-affirming hormones, and 66% of postmenopausal people reported breakthrough bleeding. We found that increased/breakthrough bleeding was significantly associated with age, systemic vaccine side effects (fever and/or fatigue), history of pregnancy or birth, and ethnicity. Generally, changes to menstrual bleeding are not uncommon or dangerous, yet attention to these experiences is necessary to build trust in medicine.”
- Le Vu et al. (2022)
- “The largest associations are observed for myocarditis following mRNA-1273 vaccination in persons aged 18 to 24 years. Estimates of excess cases attributable to vaccination also reveal a substantial burden of both myocarditis and pericarditis across other age groups and in both males and females.”
- “The risk of myocarditis was substantially increased within the first week post vaccination in both males and females (Fig. 1 and Table S2). Odds-ratios associated with the second dose of the mRNA-1273 vaccine were consistently the highest, with values up to 44 (95% CI, 22–88) and 41 (95% CI, 12–140), respectively in males and females aged 18 to 24 years but remaining high in older age groups.”
- “Odds-ratios for the second dose of the BNT162b2 vaccine tended to decrease with age, from 18 (95% CI, 9–35) and 7.1 (95% CI, 1.5–33), respectively in males and females aged 12 to 17 years, down to 3.0 (95% CI, 1.5–5.9) and 1.9 (95% CI, 0.39–9.3), respectively in males and females aged 40 to 51 years.”
- “The largest associations are observed for myocarditis following mRNA-1273 vaccination in persons aged 18 to 24 years. Estimates of excess cases attributable to vaccination also reveal a substantial burden of both myocarditis and pericarditis across other age groups and in both males and females.”
- Furer et al. (2021)
- “The objective of this report is to raise awareness of reactivation of herpes zoster (HZ) following the BNT162b2 mRNA vaccination in patients with AIIRD [autoimmune inflammatory rheumatic diseases]” (Note: “The risk of HZ infection in the AIIRD population is increased compared with the general population”)
- “The close temporal association between COVID-19 vaccination and the first reactivation of the latent zoster infection poses a question of a potential causality between both events vs a pure coincidence.”
- “The objective of this report is to raise awareness of reactivation of herpes zoster (HZ) following the BNT162b2 mRNA vaccination in patients with AIIRD [autoimmune inflammatory rheumatic diseases]” (Note: “The risk of HZ infection in the AIIRD population is increased compared with the general population”)
- Gat et al. (2022)
- Definition of time points: T0 = “pre-vaccination baseline control”; “T1, T2 and T3 – short, intermediate, and long-term evaluations, after 15-45, 75-150, and over 150 days after the vaccination date, respectively”
- “Objective: To investigate the effect of covid-19 BNT162b2 (Pfizer) vaccine on semen parameters among semen donors (SD)”
- Results:
- “15.4% sperm concentration decrease on T2 (CI -25.5%–3.9%, p=0.01) leading to total motile count 22.1% reduction (CI -35% – -6.6%, p=0.007) compared to T0.”
- The authors also report significant reductions in:
- First sample per donor in each time point (Table 3)
- Sperm concentration reduction on T2 compared to T0 (p=0.02)
- Sperm motility reduction on T2 compared to T0 (p=0.002)
- Sperm concentration reduction on T2 compared to T0 (p=0.02)
- Samples’ mean per donor in each time frame (Table 4)
- Sperm concentration reduction on T2 compared to T0 (p=0.004)
- Sperm motility reduction on T2 compared to T0 (p=0.003)
- Sperm concentration reduction on T2 compared to T0 (p=0.004)
- First sample per donor in each time point (Table 3)
- While the authors claim that “T3 [i.e. “long-term] evaluation demonstrated overall recovery. Semen volume and sperm motility were not impaired.”, Table 2 appears to indicate a substantial reduction in both Sperm concentration (15.9% reduction) and Total Motile Count (19.4% reduction). Based on the confidence intervals, while the changes are accurately reported to be non-significant, it appears that the data may have at least been tending towards significance. What this means is that the differences may have been nearly significant. One factor that could potentially impact this could be a need for a larger sample size.
- “15.4% sperm concentration decrease on T2 (CI -25.5%–3.9%, p=0.01) leading to total motile count 22.1% reduction (CI -35% – -6.6%, p=0.007) compared to T0.”
- Definition of time points: T0 = “pre-vaccination baseline control”; “T1, T2 and T3 – short, intermediate, and long-term evaluations, after 15-45, 75-150, and over 150 days after the vaccination date, respectively”
- Creutzfeldt-Jakob Disease (CJV):
- “Creutzfeldt–Jakob disease, a spongiform encephalopathy caused by prions, is characterized by a severe neurological destruction, which has an extremely high mortality.” (Kuvandık et al., 2022)
- Kuvandık et al. (2022) describe a case report of CJV following the reception of the COVID-19 vaccine. The authors report that the patient “was admitted to the Pamukkale University Anesthesiology Intensive Care Units with the neurological findings that developed after the COVID-19 vaccine (CoronaVac, Sinovac Life Sciences, Beijing, China). The patient died due to the progressive neurological disorders. In cases where rapidly progressive neurological disorders are observed, Creutzfeldt–Jakob disease should be considered and the role of immunity-related conditions in the progression of the disease should be investigated.”
- Perez et al. (2022)
- “highlight the presence of a Prion region in the different Spike proteins of the original SARS-CoV2 virus as well as of all its successive variants but also of all the “vaccines” built on this same sequence of the Spike SARS-CoV2 from Wuhan.”
- The prion region is reported to have a “a density of mutations 8 times greater than that of the rest of the spike, the possible harmfulness of this Prion region disappears completely in the Omicron variant”
- The authors also analyze “the concomitance of cases, which occurred in various European countries, between the first doses of Pfizer or Moderna mRNA vaccine and the sudden and rapid onset of the first symptoms of Creutzfeldt-Jakob disease, which usually requires several years before observing its first symptoms.”
- “In a few weeks, more 50 cases of almost spontaneous emergence of Creutzfeldt-Jakob disease have appeared in France and Europe very soon after the injection of the first or second dose of Pfizer, Moderna or AstraZeneka vaccines.”
- “To summarize, of the 26 cases analyzed, the first symptoms of CJD appeared on average 11.38 days after the injection of the COVID-19 “vaccine”. Of these 26 cases, 20 had died at the time of writing this article while 6 were still alive. The 20 deaths occurred only 4.76 months after the injection. Among them, 8 of them lead to a sudden death (2.5 months). All this confirms the radically different nature of this new form of CJD, whereas the classic form requires several decades.”
- “highlight the presence of a Prion region in the different Spike proteins of the original SARS-CoV2 virus as well as of all its successive variants but also of all the “vaccines” built on this same sequence of the Spike SARS-CoV2 from Wuhan.”
- “Creutzfeldt–Jakob disease, a spongiform encephalopathy caused by prions, is characterized by a severe neurological destruction, which has an extremely high mortality.” (Kuvandık et al., 2022)
- “An increase of over 25% was detected in both call types during January–May 2021, compared with the years 2019–2020. Using Negative Binomial regression models, the weekly emergency call counts were significantly associated with the rates of 1st and 2nd vaccine doses administered to this age group but were not with COVID-19 infection rates.” (Sun et al., 2022)
- “Results of this large cohort study indicated that both first and second doses of mRNA vaccines were associated with increased risk of myocarditis and pericarditis.” (Karlstad et al., 2022)
- “Using surveillance results from Dec 14, 2020, to Aug 31, 2021, we identified 21 individuals with MIS-C [Multisystem inflammatory syndrome in children] after COVID-19 vaccination. Of these 21 individuals, median age was 16 years (range 12–20); 13 (62%) were male and eight (38%) were female. All 21 were hospitalised: 12 (57%) were admitted to an intensive care unit and all were discharged home. 15 (71%) of 21 individuals had laboratory evidence of past or recent SARS-CoV-2 infection, and six (29%) did not. As of Aug 31, 2021, 21 335 331 individuals aged 12–20 years had received one or more doses of a COVID-19 vaccine, making the overall reporting rate for MIS-C after vaccination 1.0 case per million individuals receiving one or more doses in this age group. The reporting rate in only those without evidence of SARS-CoV-2 infection was 0.3 cases per million vaccinated individuals…Continued surveillance for MIS-C illness after COVID-19 vaccination is warranted, especially as paediatric COVID-19 vaccination is authorised and recommended for younger children who comprise the highest proportion of MIS-C cases after SARS-Cov-2 infection” (Yousaf et al., 2022)
- “Of those with myocarditis, the median age was 21 years (IQR, 16-31 years) and the median time to symptom onset was 2 days (IQR, 1-3 days). Males comprised 82% of the myocarditis cases for whom sex was reported. The crude reporting rates for cases of myocarditis within 7 days after COVID-19 vaccination exceeded the expected rates of myocarditis across multiple age and sex strata. The rates of myocarditis were highest after the second vaccination dose in adolescent males aged 12 to 15 years (70.7 per million doses of the BNT162b2 vaccine), in adolescent males aged 16 to 17 years (105.9 per million doses of the BNT162b2 vaccine), and in young men aged 18 to 24 years (52.4 and 56.3 per million doses of the BNT162b2 vaccine and the mRNA-1273 vaccine, respectively).” (Oster et al., 2022)
- Abstract: “Cases of myocarditis, diagnosed clinically by laboratory tests and imaging have been described in the context of mRNA-based anti-SARS-CoV-2 vaccination…We describe the autopsy findings and common characteristics of myocarditis in untreated persons who received anti-SARS-CoV-2 vaccination. Standardized autopsies were performed on 25 persons who had died unexpectedly and within 20 days after anti-SARS-CoV-2 vaccination. In four patients who received a mRNA vaccination, we identified acute (epi-) myocarditis without detection of another significant disease or health constellation that may have caused an unexpected death. Histology showed patchy interstitial myocardial T-lymphocytic infiltration, predominantly of the CD4 positive subset, associated with mild myocyte damage. Overall, autopsy findings indicated death due to acute arrhythmogenic cardiac failure. Thus, myocarditis can be a potentially lethal complication following mRNA-based anti-SARS-CoV-2 vaccination…” (Schwab et al., 2022)
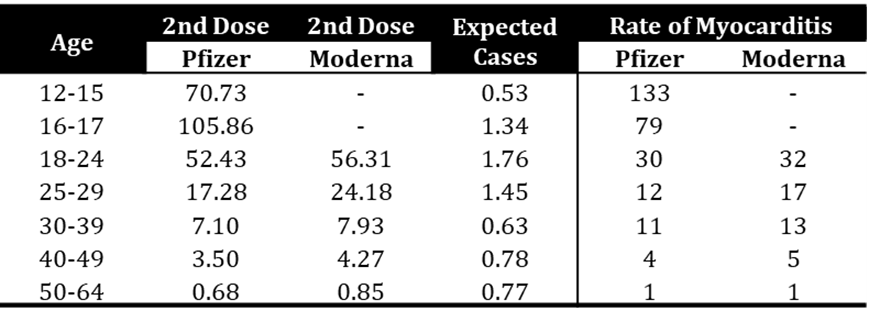
Table 3: Using data from Table 2 in Oster et al, 2022, rates of myocarditis are reported comparing 2nd dose of Pfizer and Moderna “vaccines” relative to expected rates. The number shown in the rate column reflects the number of times more that myocarditis is reported.
- “In this paper, we present the evidence that vaccination, unlike natural infection, induces a profound impairment in type I interferon signaling, which has diverse adverse consequences to human health. We explain the mechanism by which immune cells release into the circulation large quantities of exosomes containing spike protein along with critical microRNAs that induce a signaling response in recipient cells at distant sites. We also identify potential profound disturbances in regulatory control of protein synthesis and cancer surveillance. These disturbances are shown to have a potentially direct causal link to neurodegenerative disease, myocarditis, immune thrombocytopenia, Bell’s palsy, liver disease, impaired adaptive immunity, increased tumorigenesis, and DNA damage. We show evidence from adverse event reports in the VAERS database supporting our hypothesis. We believe a comprehensive risk/benefit assessment of the mRNA vaccines excludes them as positive contributors to public health, even in the context of the Covid-19 pandemic.” (Seneff et al., 2022)
- “In this systematic review and meta-analysis of 12 articles including AE [Adverse Event] reports for 45 380 trial participants, systemic AEs were experienced by 35% of placebo recipients after the first dose and 32% after the second. Significantly more AEs were reported in the vaccine groups, but AEs in placebo arms (“nocebo [negative expectations of the patient regarding a treatment lead to more negative effects from that treatment than would otherwise have been expected] responses”) accounted for 76% of systemic AEs after the first COVID-19 vaccine dose and 52% after the second dose.” [Interpretation: While the nocebo effect appears to play a role, its impact diminishes with additional vaccinations, and the authors clearly address that there are significantly more adverse events reported in the vaccine group. However, this information appears to be ignored in the conclusion of their “Key Points” section of the article, where the authors solely focused on their findings indicating that “the rate of nocebo responses in placebo arms of COVID-19 vaccine trials was substantial. Interestingly, the authors report that “Headache, fatigue, malaise, and joint pain were common in both groups and seem to have been particularly associated with nocebo.”, however these are not the side effects about which a significant concern has been expressed, given that these are side effects commonly felt after any vaccine!] (Haas et al., 2022)
- “Coronavirus disease 2019 (COVID-19) vaccination is associated with a small change in cycle length but not menses length.” (Edelman et al., 2022)
- Brock and Thornley (2021)
- “…draw attention to these errors [in the Shimabukuro (2021) paper] and recalculate the risk of this outcome based on the cohort that was exposed to the vaccine before 20 weeks’ gestation. Our re-analysis indicates a cumulative incidence of spontaneous abortion 7 to 8 times higher than the original authors’ results (p < 0.001) and the typical average for pregnancy loss during this time period.”
- “The study indicates that at least 81.9% (≥ 104/127) experienced spontaneous abortion following mRNA exposure before 20 weeks, and 92.3% (96/104) of spontaneous abortions occurred before 13 weeks’ gestation …This is a very high proportion of pregnancy loss observed in those exposed to the mRNA vaccination before 20 weeks’ gestation, ranging from 81.9–91.2%,…Considering the evidence presented here, we suggest the immediate withdrawal of mRNA vaccine use in pregnancy (Category X)[41] and those breastfeeding, alongside the withdrawal of mRNA vaccines to children or those of child-bearing age in the general population [emphasis not mine], until more convincing data relating to the safety and long-term impacts on fertility, pregnancy and reproduction are established in these groups.”
- “…draw attention to these errors [in the Shimabukuro (2021) paper] and recalculate the risk of this outcome based on the cohort that was exposed to the vaccine before 20 weeks’ gestation. Our re-analysis indicates a cumulative incidence of spontaneous abortion 7 to 8 times higher than the original authors’ results (p < 0.001) and the typical average for pregnancy loss during this time period.”
- “Ang II [angiotensin II], ACE [angiotensin-converting enzyme] 2 and Ang-(1-7) regulate follicle development and ovulation, modulate luteal angiogenesis and degeneration, and also influence the regular changes in endometrial tissue and embryo development. Taking these functions into account, 2019-nCoV may disturb the female reproductive functions through regulating ACE2.” (Jing et al., 2020) [NOTE: These findings impacting fertility and embryo development, in addition to the increasingly evident wide distribution of the mRNA-containing nanoparticles in the “vaccines”/gene therapies, appear to further corroborate the observed higher levels of spontaneous abortions, impacts on menstruation, etc. and support a potential link to increased infertility as a result of the COVID-19 “vaccines”.]
- “This retrospective case series studied patients within the US Military Health System who experienced myocarditis after COVID-19 vaccination between January and April 2021…In this case series, myocarditis occurred in previously healthy military patients with similar clinical presentations following receipt of an mRNA COVID-19 vaccine.” (Montgomery et al., 2021)
- Patone et al. (2021)
- “We estimate that the absolute number of excess myocarditis events in the 28 days following a first dose of adenovirus or mRNA vaccine is between one and six per million persons vaccinated, and the excess risk following the second dose of the mRNA-1283 vaccine is ten per million. By contrast, we estimate 40 excess myocarditis events per million in the 28 days following SARS-CoV-2 infection.” [Interpretation: there is a substantially higher chance of myocarditis following infection than vaccination. However, from my reading, while the authors indicate a balanced sample in terms of those reporting previous cardiac inflammation reports “in the previous 2 years”, there is no indication of how similar/balanced the sample was in relation to other comorbidities e.g. obesity, diabetes etc. i.e. Did the positive-SARS-CoV-2 test sample have higher numbers of people with non-cardiac related, but pre-disposing comorbidities?]
- “Our findings are relevant to the public, clinicians and policy makers. First, there was an increase in the risk of myocarditis within a week of receiving the first dose of both adenovirus and mRNA vaccines, and a higher increased risk after the second dose of both mRNA vaccines…Third, the increased risk of myocarditis after vaccination was higher in persons aged under 40 years.” [NOTE: this is a group that has an average fatality rate from COVID-19 of ~0.01% (calculated from O’Driscoll et al. (2021) taking the average of the percentages for ages less than 40)]
- “We estimate that the absolute number of excess myocarditis events in the 28 days following a first dose of adenovirus or mRNA vaccine is between one and six per million persons vaccinated, and the excess risk following the second dose of the mRNA-1283 vaccine is ten per million. By contrast, we estimate 40 excess myocarditis events per million in the 28 days following SARS-CoV-2 infection.” [Interpretation: there is a substantially higher chance of myocarditis following infection than vaccination. However, from my reading, while the authors indicate a balanced sample in terms of those reporting previous cardiac inflammation reports “in the previous 2 years”, there is no indication of how similar/balanced the sample was in relation to other comorbidities e.g. obesity, diabetes etc. i.e. Did the positive-SARS-CoV-2 test sample have higher numbers of people with non-cardiac related, but pre-disposing comorbidities?]
- “The unadjusted incidence rate of confirmed cases of GBS [Guillain-Barre syndrome] per 100,000 person-years in the 1-21 days after Ad.26.COV2.S was 34.6 (95% confidence interval [CI]: 15.8-65.7), significantly higher than the background rate, and the adjusted RR [rate ratio] in the 1-21 vs. 22-42 days following Ad.26.COV2.S [Janssen] was 6.03 (95% CI: 0.79-147.79). Thirty-four cases of GBS after mRNA vaccines were confirmed. The unadjusted incidence rate of confirmed cases per 100,000 person-years in the 1-21 days after mRNA vaccines was 1.4 (95% CI: 0.7-2.5) and the adjusted RR in the 1-21 vs. 22-42 days following mRNA vaccines was 0.56 (95% CI: 0.21-1.48). In a head-to-head comparison of Ad.26.COV2.S vs. mRNA vaccines, the adjusted RR was 20.56 (95% CI: 6.94-64.66). [Interpretation: Higher possibility of developing Guillain-Barre Syndrome with Janssen/Johnson & Johnson vaccine] (Hanson et al., 2021)
- “These findings suggest a potential small but statistically significant safety concern for Guillain-Barre syndrome following receipt of the Ad26.COV2.S vaccine.” (Woo et al., 2021) [NOTE: If the CDC can claim that masks work with a small (1.8% max) reduction in the spread of COVID-19 because it is significant, then this information relating a “small but statistically significant” concern should be given just as much attention. Also of note, given the push to vaccinate younger people, the authors note: “…the observed to expected rate ratio was elevated in all age groups except individuals aged 18 through 29 years.”
- Vaccine-induced psychosis: Flannery et al. (2021) report about a “case of anti-NMDAR encephalitis complicating SARS-CoV2 vaccination in a previously healthy young woman” that resulted in psychosis. [NOTE: Anti-NMDAR encephalitis – Background: The NMDA receptor is one of the fundamental receptors in the brain and regulate alertness, wakefulness, learning, memory etc. The fact that the natural normal receptor in the brain is being attacked reflects an auto-immune response – that is that the body’s immune system is recognizing the normal/natural receptor as a foreign body. Once thought to primarily affect adult women and associated with the presence of tumors, it has come to be recognized in children, in males and in the absence of tumors (Chapman and Vause, 2011). Given the significant role of this receptor, significant psychosis, among many other effects, can result with anything that causes it to be dysfunctional.]
- Also see letter by Dr. Patricia Lee (who is a “licensed physician practicing in the state of California”, obtained her “medical degree from University of Southern California”, received her “post-graduate training at Georgetown University and Harvard-affiliated hospitals”, doctor for more than twenty years, and is fully vaccinated) to Dr. Marks, Director of the FDA and Dr. Shimabukuro on the COVID-19 Vaccine Task Force of the CDC regarding her experience treating ICU patients and how it “does not comport with claims made by federal health authorities regarding the safety of Covid-19 vaccines” . In the letter she expresses how she feels “compelled by conscience to state the facts as I observe them on the frontlines”. Despite all her background and experience she states that she has “never witnessed so many vaccine-related injuries until this year”.
- Title: “Comprehensive investigations revealed consistent pathophysiological alterations after vaccination with COVID-19 vaccines”. In the Abstract: “…besides generation of neutralizing antibodies, consistent alterations in hemoglobin A1c, serum sodium and potassium levels, coagulation profiles, and renal functions in healthy volunteers after vaccination with an inactivated SARS-CoV-2 vaccine. Similar changes had also been reported in COVID-19 patients, suggesting that vaccination mimicked an infection…our study recommends additional caution when vaccinating people with pre-existing clinical conditions, including diabetes, electrolyte imbalances, renal dysfunction, and coagulation disorders.” (Liu et al., 2021a) [Interpretation: vaccine causing similar problems to disease]
- “Thus, for 6 (95% CI 2–11) deaths prevented by vaccination, there were approximately 4 deaths reported to Dutch Lareb that occurred after vaccination, yielding a potential risk/benefit ratio of 2:3. Conclusion: Although causality between ICSRs and vaccination has not been established, these data indicate a lack of clear benefit, which should cause governments to rethink their vaccination policy.” (Walach et al., 2021a)
[NOTE: The Walach paper was originally published in the journal Vaccines, following peer review. A few days after it was published (seems rather fast), the paper was retracted. The retraction statement is shown in the image below (B). There is a significant element, in the retraction, that appears to be unprofessional and quasi-colloquial – certainly not the way you would write in a scientific journal. Additionally, it is clear that the biggest issue the editors had was the potentially implied causal relationship between the vaccine and the adverse events reported through the ICSR (Individual Case Safety Reports). The editors state that the authors “were not able to” respond “satisfactorily” to the request for a response, and as a result the paper was retracted.
The authors then published the manuscript in Science, Public Health Policy and the Law (which is not peer reviewed). You will note, as quoted above and seen in the image below (C), that it does not appear that the authors had any problems making a statement advising caution in relation to implying causality. So, the question is what is really going on here? Are the scientific journals censoring out what does not fit a specific narrative instead of seeking to investigate/address the truth?
- “In this systematic review and meta-analysis of 12 articles including AE [Adverse Event] reports for 45 380 trial participants, systemic AEs were experienced by 35% of placebo recipients after the first dose and 32% after the second. Significantly more AEs were reported in the vaccine groups, but AEs in placebo arms (“nocebo [negative expectations of the patient regarding a treatment lead to more negative effects from that treatment than would otherwise have been expected] responses”) accounted for 76% of systemic AEs after the first COVID-19 vaccine dose and 52% after the second dose.” [Interpretation: While the nocebo effect appears to play a role, its impact diminishes with additional vaccinations, and the authors clearly address that there are significantly more adverse events reported in the vaccine group. However, this information appears to be ignored in the conclusion of their “Key Points” section of the article, where the authors solely focused on their findings indicating that “the rate of nocebo responses in placebo arms of COVID-19 vaccine trials was substantial. Interestingly, the authors report that “Headache, fatigue, malaise, and joint pain were common in both groups and seem to have been particularly associated with nocebo.”, however these are not the side effects about which a significant concern has been expressed, given that these are side effects commonly felt after any vaccine!] (Haas et al., 2022)
Figure 11: Walach et al, 2021 – (A) original publication information in the journal Vaccines. (B) publication date of retraction and retraction. (C) Conclusion statement from Walach et al, 2021, now published in Science, Public Health Policy and the Law.
- Data Sources:
- In addition, the following website may also be of assistance in regards to adverse drug reaction reports: http://www.vigiaccess.org/
- 1) 2,219,299 reports of adverse events from the COVID-19 vaccines have been reported (with the majority in 2021) – data accessed 10/10/2021
- 2) Indicates higher risks of various side effects addressed below, from the vaccines.
- 1) 2,219,299 reports of adverse events from the COVID-19 vaccines have been reported (with the majority in 2021) – data accessed 10/10/2021
- “VAERS [Vaccine Adverse Event Reporting System] data are limited to vaccine adverse event reports received between 1990 and the most recent date” [NOTE: However, COVID vaccines became available ~late 2020/early 2021].
- 1) Data can be downloaded from: https://wonder.cdc.gov/vaers.html
- 1) Data can be downloaded from: https://wonder.cdc.gov/vaers.html
- In addition, the following website may also be of assistance in regards to adverse drug reaction reports: http://www.vigiaccess.org/
- Percentages shown are COVID-19-vaccine-related reports as a percentage of all reports of the specific event for the listed vaccines included in the comparison/data extraction:
- Amenorrhea (absence of a menstrual period in a woman of reproductive age) and Dysmenorrhea (pain during menstruation): 80% [“These results are for 1,823 total events.”]; ALL vaccines [Grouped by Vaccine type & not distinguishing between various manufacturers; Date of extraction: 09/17/21]
- These reports are not restricted to the US. “Changes to periods and unexpected vaginal bleeding are not listed, but primary care clinicians and those working in reproductive health are increasingly approached by people who have experienced these events shortly after vaccination. More than 30 000 reports of these events had been made to MHRA’s yellow card surveillance scheme for adverse drug reactions by 2 September 2021, across all covid-19 vaccines currently offered.”(Male, 2021b)
- Knowledge in this field is, as in regards to most aspects relating to the COVID-19 vaccines, is significantly lacking “Although reported changes to the menstrual cycle after vaccination are short lived, robust research into this possible adverse reaction remains critical to the overall success of the vaccination programme…We are still awaiting definitive evidence…” (Male, 2021b)
- NOTE: please keep in mind that the normal menstruation is an inflammatory process which involves the immune system. Changes in the immune system function can lead to disturbances in the menstrual cycle (Berbic and Fraser, 2013).
- See above under “Potential toxicity of nanoparticles used in drug delivery” under “Potential for side effects beyond site of injection” in relation to potential impact on male fertility.
- These reports are not restricted to the US. “Changes to periods and unexpected vaginal bleeding are not listed, but primary care clinicians and those working in reproductive health are increasingly approached by people who have experienced these events shortly after vaccination. More than 30 000 reports of these events had been made to MHRA’s yellow card surveillance scheme for adverse drug reactions by 2 September 2021, across all covid-19 vaccines currently offered.”(Male, 2021b)
- Cardiac-related events: 92% [Comparison to DTAP, Hep B, MMR; Date of extraction: 06/02/21]
- Pericarditis; Myocarditis: 72% [Comparison to all vaccines; Date of extraction: 09/25/21]
- Despite the fact that these side effects have also been reported for other vaccines e.g. the tetanus vaccine (Dilber et al., 2003), the relative occurrence is significantly higher for the COVID-19 vaccines.
- “These findings suggest a markedly higher risk for myocarditis subsequent to COVID-19 injectable product use than for other known vaccines, and this is well above known background rates for myocarditis. COVID-19 injectable products are novel and have a genetic, pathogenic mechanism of action causing uncontrolled expression of SARS-CoV-2 spike protein within human cells. When you combine this fact with the temporal relationship of AE [Adverse Event] occurrence and reporting, biological plausibility of cause and effect, and the fact that these data are internally and externally consistent with emerging sources of clinical data, it supports a conclusion that the COVID-19 biological products are deterministic for the myocarditis cases observed after injection” (Rose and McCullough, 2021) [NOTE: currently listed by Retraction Watch as Temporarily Removed]
- Additionally, when split by age the rates are as follows, indicating a higher vulnerability for younger recipients of the vaccine:
6-17 years 18.20%
18-29 years 27.86%
30-39 years 12.69%
40-49 years 8.98%
50-59 years 8.28%
60-64 years 4.04%
65-79 years 7.15%
80+ years 1.07%
Unknown 4.71% - Other literature relating to myocarditis
- “The incidence of myocarditis, although low, increased after the receipt of the BNT162b2 vaccine, particularly after the second dose among young male recipients.” (Mevorach et al., 2021)
- Other studies also report incidences of myocarditis and other side effects resulting from the vaccine BNT162b2 (Barda et al., 2021; Witberg et al., 2021) and higher incidences in younger males (Witberg et al., 2021). The Barda study (2021) reports significantly higher negative effects from SARS-CoV-2 infection in the unvaccinated:
- risk ratio for myocarditis in vaccinated of 3.24 (95% confidence interval [CI], 1.55 to 12.44) [risk ratio (RR) “tells us how many times more likely the outcome occurs among people with the risk factor (or exposure)” (Viera, 2008) – for interpretation multiply by 100 i.e. 324 times more likely to occur];
- risk ratio for myocarditis following SARS-CoV-2 infection of 18.28 (95% CI, 3.95 to 25.12).
- risk ratio for myocarditis in vaccinated of 3.24 (95% confidence interval [CI], 1.55 to 12.44) [risk ratio (RR) “tells us how many times more likely the outcome occurs among people with the risk factor (or exposure)” (Viera, 2008) – for interpretation multiply by 100 i.e. 324 times more likely to occur];
- ISSUE: Study removes people with the combination of SARS-CoV-2 infection + Vaccination (“In the vaccination analysis, so as not to attribute complications arising from SARS-CoV-2 infection to the vaccination (or lack thereof), we also censored data on the matched pair if and when either member received a diagnosis of SARS-CoV-2 infection. Similarly, in the SARS-CoV-2 infection analysis, we censored data on the matched pair if and when either member was vaccinated.”). Therefore, the study does not address the likelihood of events (e.g. myocarditis) in those vaccinated AND infected (i.e. in the case of breakthrough infections).
- This is a follow up to the Barda et al. (2021) study mentioned above. The authors were requested to further breakdown and analyze the data by age and sex. This was provided in a letter to the editor of the NEJM, in addition to supplementary documents. The issue addressed above in relation to the paper remains: “After vaccination, the risk was increased mostly among young male adolescents and adults (16 to 39 years of age), with 8.62 excess events per 100,000 persons (95% confidence interval [CI], 2.82 to 14.35). After infection, the risk was increased in both age categories (<40 and ≥40 years) and in both male and female adolescents and adults, with 11.54 excess events per 100,000 persons (95% CI, 2.48 to 22.55) in young male adolescents and adults.” (Dagan et al., 2021) [NOTE: In relation to confidence intervals (CI), the larger the CI for a particular estimate, the more caution is necessary when using the estimate.]
- “The incidence of myocarditis, although low, increased after the receipt of the BNT162b2 vaccine, particularly after the second dose among young male recipients.” (Mevorach et al., 2021)
- Despite the fact that these side effects have also been reported for other vaccines e.g. the tetanus vaccine (Dilber et al., 2003), the relative occurrence is significantly higher for the COVID-19 vaccines.
- Any adverse event: 66% [Comparison to Flu vaccines; Date of extraction: 06/04/21]
- Stroke: 89% [Comparison to DTAP, Hep B, MMR, Flu; Date of extraction: 06/22/21]
- Spontaneous Abortion: 53% [Comparison to all vaccines; Date of extraction: 09/20/21]
- This contradicts the reports of safety in pregnant women (Male, 2021a)
- Additionally, see Pfizer study (Pfizer) above, which indicates the potential for the vaccine lipid nanoparticles to be concentrated in the ovaries (among other organs).
- Shimabukuro et al. (2021) and editorial comments by Riley (2021) on the same paper report:
- Adverse neonatal outcomes included preterm birth (9.4%)
- Small size for gestational age (3.2%)
- Congenital abnormalities (2.2%)
- Pregnancy losses (13.9%)
- ISSUE: Changes to original paper Shimabukuro et al. (2021) in relation to spontaneous abortion are interesting:
- Original paper reports 12.6% (104/827) spontaneous abortions
- Correction states: “the “V-safe Pregnancy Registry” cell should have read “104,” rather than “104/827 (12.6)‡” and the Associated double dagger footnote states “No denominator was available to calculate a risk estimate for spontaneous abortions, because at the time of this report, follow-up through 20 weeks was not yet available for 905 of the 1224 participants vaccinated within 30 days before the first day of the last menstrual period or in the first trimester. Furthermore, any risk estimate would need to account for gestational week–specific risk of spontaneous abortion.”
- Original paper reports 12.6% (104/827) spontaneous abortions
- ISSUE: Changes to editorial comments by Riley (2021) are also interesting (bolded information was removed in correction):
- Original editorial reports: “…a completed pregnancy, the pregnancy resulted in a spontaneous abortion in 104 (12.6%) and in stillbirth in 1 (0.1%); these percentages are well within the range expected as an outcome for this age group of persons whose other underlying medical conditions are unknown.”
- Correction states: “Among 827 registry participants who reported a completed pregnancy, 104 experienced spontaneous abortions and 1 had a stillbirth,”
- Original editorial reports: “…a completed pregnancy, the pregnancy resulted in a spontaneous abortion in 104 (12.6%) and in stillbirth in 1 (0.1%); these percentages are well within the range expected as an outcome for this age group of persons whose other underlying medical conditions are unknown.”
- Additionally, of significance for the low percentage from which the authors obtained their numbers, the authors also note that “only a small fraction (4.7%) have enrolled in the v-safe pregnancy registry”
- ISSUE: Suggestion to violate human rights? “This situation underscores the urgent need not only to include pregnant women in clinical trials,” (Riley, 2021). Given the absence of sufficient pre-clinical investigation and evidence (i.e. experiments in animals), this suggestion is in violation of:
- Section 45 of the Code of Federal Regulations on the Protection of Human Subjects: “§ 46.204 Research involving pregnant women or fetuses: Pregnant women or fetuses may be involved in research if all of the following conditions are met: (a) Where scientifically appropriate, preclinical studies, including studies on pregnant animals, and clinical studies, including studies on nonpregnant women, have been conducted and provide data for assessing potential risks to pregnant women and fetuses;”
- The Helsinki Declaration section 21: “Medical research involving human subjects must conform to generally accepted scientific principles, be based on a thorough knowledge of the scientific literature, other relevant sources of information, and adequate laboratory and, as appropriate, animal experimentation.”
- The Nuremberg Code: “The experiment should be so designed and based on the results of animal experimentation and a knowledge of the natural history of the disease or other problem under study that the anticipated results justify the performance of the experiment.”
- Section 45 of the Code of Federal Regulations on the Protection of Human Subjects: “§ 46.204 Research involving pregnant women or fetuses: Pregnant women or fetuses may be involved in research if all of the following conditions are met: (a) Where scientifically appropriate, preclinical studies, including studies on pregnant animals, and clinical studies, including studies on nonpregnant women, have been conducted and provide data for assessing potential risks to pregnant women and fetuses;”
- Adverse neonatal outcomes included preterm birth (9.4%)
- Spontaneous abortion is also the highest reported adverse event (58%) for the COVID-19 vaccines within the section “Pregnancy, puerperium and perinatal conditions” on the VigiAccess™ website (accessed 10/10/2021)
- This contradicts the reports of safety in pregnant women (Male, 2021a)
- 28.6% of ALL side effects reported [“These results are for 1,130,123 total events.”]; ALL vaccines [Grouped by Vaccine type & not distinguishing between various manufacturers: 95 vaccines; Date of extraction: 07/17/21]
- Amenorrhea (absence of a menstrual period in a woman of reproductive age) and Dysmenorrhea (pain during menstruation): 80% [“These results are for 1,823 total events.”]; ALL vaccines [Grouped by Vaccine type & not distinguishing between various manufacturers; Date of extraction: 09/17/21]
- 7.12 Autoimmune disease
- Definition: “The diverse immune system developed to fulfil the primary function of protecting hosts from infectious agents…The failure to distinguish self from nonself is often termed a breach of tolerance and is the basis for autoimmune disease” (Wang et al., 2015)
- With time we are seeing an increase in reports of a dysfunctional immune system (e.g., cancer, Type I diabetes) following COVID-19 “vaccination”. Some of these reports are addressed in this section and include, but are not limited to, autoimmune disorders.
- SARS-CoV-2 has been reported to trigger an autoimmune response (Liu et al., 2021b)
- COVID-19 vaccine reported to be potentially linked to autoimmune disorder Guillain-Barré syndrome (immune cells attack nervous system) (Dyer, 2021)
- Merchant (2021) in a Rapid Response to the editor relating to the Dyer paper reports that:
- “Study 514559 showed that the Covid vaccine AZ was distributed to sciatic nerves in almost all animals and the distributed fractions did not clear throughout the study. The last sample was taken on 29 days post-administration and sciatic nerves of 70% of animals were still tested positive at the end of the study. The vaccine distribution to the sciatic nerves may lead to conditions like sciatica that has been previously linked to the viral infection of the sciatic nerve, such as herpes.”
- “The biodistribution of the vaccine to other nerves is not known as the study 514559 checked for sciatic nerves only being anatomically closer to the injection site (hind limb) in mice. The facial(cranial) nerves, on the contrary, are anatomically closer to the vaccine injection site in humans (deltoid muscle).” While the response does not directly make the direct link, it does state that “The MHRA database listed ~1031 cases of facial cranial nerve disorders (527cases of Bell’s palsy and 457 cases of facial paresis/paralysis), 20 cases of Miller Fisher syndrome [similar to Guillain-Barre syndrome] and additional 372 cases of Guillain-Barre syndrome (2 fatal) following AZ vaccine up until 28th July 2021.”
- “The biodistribution (study 514559) also evidenced the vaccine distribution via blood circulation to other tissues notably bone marrow, liver, mammary glands and spleen. The vaccine encoded gene transfection to distant tissues is likely to attract an immune response against various body tissues that can manifest into various autoimmune conditions.”
- “These autoimmune responses may well be transient in many healthy subjects, and the immune response is likely to be very selective towards vaccine transfected cells only, however, the possibility of developing a chronic autoimmune condition in some individuals cannot be overruled”
- “Study 514559 showed that the Covid vaccine AZ was distributed to sciatic nerves in almost all animals and the distributed fractions did not clear throughout the study. The last sample was taken on 29 days post-administration and sciatic nerves of 70% of animals were still tested positive at the end of the study. The vaccine distribution to the sciatic nerves may lead to conditions like sciatica that has been previously linked to the viral infection of the sciatic nerve, such as herpes.”
- “The reactogenicity of COVID-19 mRNA vaccine in individuals suffering from immune-mediated diseases and having therefore a pre-existent dysregulation of the immune response has not been investigated.” (Talotta, 2021)
- “…we hypothesize that, even though, COVID-19 vaccination does not provoke de novo immune mediated adverse events, it is possible that, the immunologic response triggers pre-existing underlying dysregulated pathways.” (Akinosoglou et al., 2021)
- “Vaccine-associated autoimmunity is a well-known phenomenon attributed to either the cross-reactivity between antigens or the effect of adjuvant [3]. When coming to COVID-19 vaccine, this matter is further complicated by the nucleic acid formulation and the accelerated development process imposed by the emergency pandemic situation [4].” (Talotta, 2021)
- Vojdani and Kharrazian (2020)
- “There are reasons for all the precautions involved in developing a vaccine, not the least of which are unwanted side-effects. In light of the information discussed above about the cross-reactivity of the SARS-CoV-2 proteins with human tissues and the possibility of either inducing autoimmunity, exacerbating already unhealthy conditions, or otherwise resulting in unforeseen consequences, it would only be prudent to do more extensive research regarding the autoimmune-inducing capacity of the SARS-CoV-2 antigens.”
- “…our own findings that 21 out of 50 tissue antigens had moderate to strong reactions with the SARS-CoV-2 antibodies are a sufficiently strong indication of cross-reaction between SARS-CoV-2 proteins and a variety of tissue antigens beyond just pulmonary tissue, which could lead to autoimmunity against connective tissue and the cardiovascular, gastrointestinal, and nervous systems.”
- “There are reasons for all the precautions involved in developing a vaccine, not the least of which are unwanted side-effects. In light of the information discussed above about the cross-reactivity of the SARS-CoV-2 proteins with human tissues and the possibility of either inducing autoimmunity, exacerbating already unhealthy conditions, or otherwise resulting in unforeseen consequences, it would only be prudent to do more extensive research regarding the autoimmune-inducing capacity of the SARS-CoV-2 antigens.”
- “This letter addresses the issue of why SARS-CoV-2 attacks the respiratory system and reports on a vast peptide sharing between SARS-CoV-2 spike glycoprotein and surfactant-related proteins… results suggest that immune responses following SARS-CoV-2 infection might lead to cross-reactions with pulmonary surfactant and related proteins, and might contribute to the SARS-CoV-2-associated lung diseases. The data warn against using vaccines based on entire SARS-CoV-2 antigens to fight SARS-CoV infections, and highlight peptide uniqueness as a molecular concept for effective anti-CoV immunotherapy” (Kanduc and Shoenfeld, 2020)
- Definition: “The diverse immune system developed to fulfil the primary function of protecting hosts from infectious agents…The failure to distinguish self from nonself is often termed a breach of tolerance and is the basis for autoimmune disease” (Wang et al., 2015)
- 7.13 Additional concerns
- Reiken et al. (2022) report:
- “Discussion: COVID-19 neuropathology includes AD-like [Alzheimer’s Disease-Like] features and leaky RyR2 channels could be a therapeutic target for amelioration of some cognitive defects associated with SARS-CoV-2 infection and long COVID.”
- [Comments/Questions: Given the conclusion of the authors as well as considering:
1) the material addressed in various sections of this document,
2) what we know about the pathology of COVID,
3) the role of the spike protein in the disease including:
- its interaction with the ACE2 receptor (Kuhn et al., 2004;Jackson et al., 2021;Xia, 2021) which can impact mental health (Raony et al., 2020)
- its capacity to alter the barrier function of the blood-brain barrier (Buzhdygan et al., 2020;Zhang et al., 2021c)
- its capacity to cross the blood-brain barrier (Rhea et al., 2021;Zhang et al., 2021c)
- its capacity to impact neurodegeneration, through the inflammatory response, through a common pathway impacted by implemented measures such as social isolation etc., potentially exacerbating neuronal degeneration (Raony et al., 2020)
- its interaction with the ACE2 receptor (Kuhn et al., 2004;Jackson et al., 2021;Xia, 2021) which can impact mental health (Raony et al., 2020)
- Why…
- Administer viral genetic material encapsulated in nanoparticles that have the potential to cross the blood-brain barrier (Wang et al., 2018) and the potential to damage nerves (Merchant, 2021)?
- Consider and investigate the use of administering the “vaccines”/gene therapies via the nasal route (using a spray) (Rubin, 2021), especially when we know that this route is able to bypass the blood-brain barrier (Wang et al., 2019) and therefore increase the risk of detrimental consequences on the central nervous system?
- ….and so many more questions.
- Administer viral genetic material encapsulated in nanoparticles that have the potential to cross the blood-brain barrier (Wang et al., 2018) and the potential to damage nerves (Merchant, 2021)?
- “Discussion: COVID-19 neuropathology includes AD-like [Alzheimer’s Disease-Like] features and leaky RyR2 channels could be a therapeutic target for amelioration of some cognitive defects associated with SARS-CoV-2 infection and long COVID.”
- Reiken et al. (2022) report: